Abstract
Purpose
To report outcome of eyes with recalcitrant and naive eyes with diabetic macular edema (DME) treated with intravitreal dexamethasone implants (Ozurdex) injection.
Methods
Retrospective multicenter data analysis of eyes with DME treated with Ozurdex implant and with minimum follow-up of at least one year after the first implant. Data collected included demographic details, history of presenting illness, past treatment history, clinical examination details including visual acuity at presentation, and follow-up with imaging and treatment details. Paired sample t-test was used to measure mean differences between pre- and post-implant values obtained at baseline and last follow-up.
Results
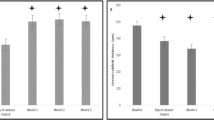
A total of 79 eyes (62 subjects) were included. Sixty-four eyes had been previously treated; 15 eyes were naive. Among the previously treated eyes, mean interval between first Ozurdex injection and any previous treatment was 7.69±8.2 months. In naive eyes, the visual acuity improved from baseline 0.58±0.25 to 0.44±0.33 logMAR at last follow-up (P=0.05). In eyes that had been previously treated, the improvement was from 0.65±0.34 at baseline to 0.48±0.35 logMAR (P=0.01). Mean treatment-free interval was 6.5±4.5 months. Nine eyes were steroid responder with controlled intraocular pressure (IOP), none showed any spike in IOP during the follow-up period.
Conclusions
Ozurdex implant could be a good alternative for recalcitrant as well as naive eyes with DME. The visual gain after initial implant injection was fairly maintained, with additional treatment usually after 6 months in naive eyes. Ozurdex appeared safe even in steroid responders with good control of IOP with antiglaucoma medications.
Similar content being viewed by others
Introduction
Various treatment modalities have been tried in management of diabetic macular edema (DME) including laser photocoagulation, intravitreal triamcinolone acetonide (IVTA), and antivascular endothelial growth factor (VEGF) therapy. Laser photocoagulation, the first-line treatment suggested by early-treatment diabetic retinopathy study (ETDRS), has recently taken a step back. The Diabetic Retinopathy Clinical Research (DRCR) Network protocol (I) reported good results with IVTA injections,1 however, the side effects of cataract progression and increase in intraocular pressure (IOP) limit its use in clinical practice. Limitations of fluocinolone acetonide vitreous inserts include increase in IOP and its cost, which limits its use in clinical practice.2 Anti-VEGF injections with or without laser photocoagulation has become first-line treatment of DME. However, the limitations of anti-VEGF injections include frequent injections, induction of resistance, and tachyphylaxis due to the long-term nature of the treatment.3 Cases of DME that do not respond well to regular anti-VEGF injections may be driven by proinflammatory cytokines other than VEGF.4
Most recently (June 2014), the FDA approved Ozurdex for the treatment of DME in the general population.5 However, the European Union’s Committee for Medicinal Products for Human Use (CHMP) has recommended extending the marketing authorization for Ozurdex to treat adult patients with vision loss due to DME who are pseudophakic or who are considered insufficiently responsive to or unsuitable for noncorticosteroid therapy.
Recent clinical trials PLACID and MEAD reported efficacy of Ozurdex with or without laser photocoagulation in management of DME.5, 6 However, these trials had fixed retreatment interval, and thus the efficacy of this implant at shorter interval could not be assessed. It would be interesting to evaluate the results from clinical trials in routine clinical practice. Moreover, the comparison between the efficacy of this implant in resistant and naive cases for longer follow-up has not been reported before.
This multicenter retrospective study reports treatment outcome in recalcitrant and naive eyes with DME.
Materials and methods
This is a retrospective, multicenter, data collection of interventional case series of eyes with a diagnosis of DME treated with intravitreal Ozurdex injection. The case reports came from many countries of several regions of the world (Europe, Middle East, Asia, South America). Patients examined between January 2013 to December 2013, were enrolled in the study. Institutional Review Board approval was obtained at each participating center; in addition, the study adhered to the tenets of the Declaration of Helsinki.
Data collected included demographic details, history of presenting illness, eye laterality, ocular and systemic co-morbidities, past treatment history, clinical examination details including visual acuity at presentation and follow-up with imaging and treatment details. Inclusion criteria were: (1) adults (older than equal to 18 years) controlled diabetes (HbA1c <8%); (2) the presence of center-involving DME in the study eye; (3) best-corrected visual acuity (BCVA) between 20/200 and 20/25; (4) central macular thickness (CMT) >250 microns as measured by spectral domain optical coherence tomography (SD-OCT); (5) follow-up of at least 1 year after the first Ozurdex injection was administred; and (6) availability of complete medical records including BCVA and SD-OCT throughout the follow-up. Exclusion criteria were: (1) subjects with <1 year follow-up after first Ozurdex injection; (2) unavailability of SD-OCT parameters; (3) any other significant concurrent ocular disease in the study eye, which could be the cause of vision loss.
All patients received comprehensive eye examination including color fundus images, fundus fluorescein angiography (FFA), and SD-OCT. BCVA was measured using Snellen’s charts. Previous tretaments consisted of intravitreal injection of 1.25 mg per 0.05 ml of bevacizumab (Avastin; Genentech, South San Francisco, CA, USA) or 0.5 mg per 0.05 ml of ranibizumab (Lucentis; Genentech, South San Francisco, CA, USA). After obtaining informed consent, Ozurdex (Allergan, Irvine, CA, USA) injection was given as per standard protocol. Postoperative period was uneventful in all patients. At each follow-up, SD-OCT scans were repeated. Retreatment was performed in case of fovea-involving intraretinal and/or subretinal fluid found during clinical examination and SD-OCT and/or in case of CMT higher than 250 microns on SD-OCT scan. The treatment options were discussed with the subjects. Focal laser and modified laser photocoagulation was performed as per standard ETDRS protocol as per physician’s discretion.7
Statistical analysis
Snellen’s visual acuity values were converted into logMAR for statistical analysis at baseline and the last follow-up. Descriptive statistics included mean and SD for continuous variables. Paired sample t-test was used to measure mean differences between pre- and post-implant values of all the parameters evaluated and obtained at first and the last follow-up visits. Statistical analyses were performed using commercial software (Stata data analysis and statistical software, version 12.1, StataCorp, College Station, TX, USA). A P-value of <0.05 was considered statistically significant.
Results
A total of 102 eyes of 85 subjects who received Ozurdex for DME were evaluated. Twenty-three eyes, which had <1 year follow-up, were excluded. Finally, 79 eyes of 62 subjects were included in this study. Forty-five subjects were males and 17 were females. Among different ethinicity, 18 subjects were Caucasians, 42 were Indians, and remaining two were from Middle East. Seventeen subjects had bilateral involvement. Mean duration of diabetes among subjects was 12.3±8.4 years. Different grades of diabetic retinopathy among study subjects included mild nonproliferative diabetic retinopathy (NPDR) in 4 eyes (5%), moderate NPDR in 25 eyes (31.6%), severe NPDR in 17 eyes (21.5%%), PDR in 7 eyes (8.8%), and lasered proliferative diabetic retinopathy in 26 eyes (33%). Twenty-six eyes had undergone panretinal photocoagulation, a minimum of 4 months before the first Ozurdex implant was administered. Sixty-four eyes (81%) had been previously treated for DME, whereas 15 eyes (19%) were naive. Among the 64 eyes, 55 eyes (86%) underwent anti-VEGF treatment, and 33 eyes (51.5%) underwent additional laser grid photocoagulation for DME. Ten eyes had additional intravitreal triamcinolone. Six eyes (7.6%) were steroid responders with IOP rise up to X, which was well controlled with topical antiglaucoma medications.
Mean IOP at baseline and at last follow-up was 14.3±3.2 and 15.3±2.8 mm Hg, respectively. At last follow-up, only 3 eyes were on antiglaucoma medications. Mean treatment-free interval among naive eyes and previously treated eyes was 10.53±7.8 and 6.5±4.5 months, respectively. Overall, the mean treatment-free interval was 6.5±4.5 months.
At presentation, lens was clear in 16 eyes (20%), Grade 1 Nuclear sclerosis was present in 9 eyes (11.3%), Grade 2 Nuclear sclerosis in 23 eyes (29%), and 31 eyes (39.2%) were pseudophakic. At last follow-up, lens was clear in 14 eyes (17.7%), Grade 1 Nuclear sclerosis was present in 7 eyes (8.8%), Grade 2 Nuclear sclerosis in 23 eyes (29.1%), and 35 eyes (44.3%) were pseudophakic.
At presentation, a neurosensory detachment was present in 9 eyes (11.3%), cystoid changes in the inner retina were present in 25 eyes (31.6%) and 45 eyes (56.9%) had diffuse retinal edema. None of the eyes had epiretinal membrane or vitreomacular traction. No relationship was found between Ozurdex response and the type of edema on OCT.
During the mean follow-up period of 18.3±6.1 months, 57 (72%) eyes not required any additional treatment. Total number of Ozurdex injections given were 1.27±0.6 during the follow-up. Total number of additional anti-VEGF injections required due to poor response to Ozurdex was 1±2.3. One eye underwent pars plana vitrectomy with internal limiting membrane peeling for non-resolving DME. Rebound phenomenon (>20% increase from baseline) was noted in four eyes at 6 months and two eyes at 12 months.
Comparison between the naive eyes and previously treated eyes are shown as Table 1. There was significant difference (P=0.016) between the longest treatment-free interval between the groups that is, 10.5±7.8 and 7.0±4.4 months among naive and previously treated eyes, respectively.
Discussion
In contrast to trials that show a required reinjection of Ozurdex at 6 months, our results show that only four eyes (5%) required additional treatment within 3 months and 17 (21.5%) eyes required additional treatment before 6 months. All 17 eyes that were retreated within 6 months had recalcitrant edema. Therefore, the results from the clinical trials are difficult to be implied on routine clinical practice.
While comparing the previously treated eyes and naive eyes, we found that both groups had improvement in visual acuity and decrease in foveal thickness. However, the percentage of eyes that required additional treatment during the follow-up period was 33.3% and 43.7% among naive and previously treated eyes, respectively. Mean follow-up period was 11±6.9 and 7.6±5.8 months among naive and previously treated eyes, respectively. In regards to CMT, there was a significant difference at the last follow-up from baseline in naive eyes (P=0.005), as well as in previously treated eyes (P=0.01). Barranco et al compared response with treatment between refractory and treatment-naive groups. They found a similar decrease in central macular thickness in both refractory and treatment-naive groups but they report a higher improvement in visual acuity in treatment-naive group.8
In our study, nine eyes were steroid responder, however, the IOP was under control with topical antiglaucoma medication before administration of first Ozurdex implant. None of these eyes had any spike in IOP during the follow-up period. Similar phenomenon has been reported by Gomez et al where they evaluated effect of Ozurdex injections on IOP in patients with intermediate and posterior uveitis, macular edema secondary to retinal vein occlusion and diabetic macular edema over a period of 6 months. No statistically significant differences were observed in IOP measurements at the follow-up visits between patients with and without a history of glaucoma.9 These findings suggests that Ozurdex can be safely used in eyes with history of steroid response safely, but with regular IOP check is mandatory. The possible explanation given is that dexamethasone, triamcinolone acetonide (TA), and fluocinolone acetonide activate different patterns of gene expression in human trabecular meshwork. Although dexamethasone is a more potent glucocorticoid but it is less lipophilic than TA or fluocinolone acetonide and does not accumulate much in the trabecular meshwork and around the lens, hence, there is decreased risk of secondary glaucoma and cataract progression with dexamethasone.10 Although in contrast to this hypothesis, Meyer et al found significant rise in IOP after intravitreal Ozurdex injection in patients with CRVO.11 According to the authors, the cause for this IOP rise could be that prolonged exposure of intraocular structures to the dexamethasone implant exceeds a dose-threshold that leads to alterations in the trabecular meshwork causing secondary outflow obstruction especially after repeated injections of the implant. We did not notice such a phenomenon even in our patients who received more than one Ozurdex injections. This may be due to the fact that CRVO is already a high-pressure state and patients with glaucoma are more prone to CRVO and vice versa,12 hence, there is relatively more risk of IOP rise in these patients with dexamethasone implant.
Fourteen (17.7%) eyes out of 79 eyes received more than one Ozurdex injection. Thirteen out of 14 eyes were previously treated with other modalities, suggesting the recalcitrant nature of the macular edema. Only one eye required modified grid laser for non-resolving macular edema at 3 months. However, 8 eyes required additional injection in the form of Ozurdex (7 eyes) and intravitreal bevacizumab (1 eye) at 6 months. In this group the BCVA improved from 0.65±0.34 logMAR (Snellen equivalent 20/100) at baseline to 0.48±0.35 logMAR (Snellen equivalent 20/60) at last follow-up. The gain in visual acuity was maintained with multiple Ozurdex injections. None of these eyes had increase in IOP during the follow-up.
In our study, only two eyes underwent cataract surgery. None of the eyes underwent glaucoma-related procedures such as laser trabeculoplasty or filtering surgery during follow-up.
The main limitation of our study includes its retrospective nature. Owing to multiple participating centers and physicians there was no standard protocol for treatment or follow-up regimen. Owing to its retrospective nature, the evaluation could not be done at specific time points. The strengths of this study include longer follow-up and assessment of efficacy of implant with addition to laser or anti-VEGF injections in real-life situations.
In conclusion, we propose that Ozurdex implant could be a good alternative for recalcitrant as well as naive eyes with DME. The gain in vision after initial implant injection was fairly maintained, with additional treatment usually after 6 months in naive eyes. Ozurdex appeared safe even in steroid responders with good control of IOP with antiglaucoma medications.

References
Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010; 117 (6): 1064–1077 e1035.
Pearson PA, Comstock TL, Ip M, Callanan D, Morse LS, Ashton P et al. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology 2011; 118 (8): 1580–1587.
Stewart MW . Critical appraisal of ranibizumab in the treatment of diabetic macular edema. Clin Ophthalmol 2013; 7: 1257–1267.
Abcouwer SF . Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol 2013; Suppl 1 (11). doi:10.4172/2155-9899.
Boyer DS, Yoon YH, Belfort R Jr, Bandello F, Maturi RK, Augustin AJ et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014; 121 (10): 1904–1914.
Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013; 120 (9): 1843–1851.
Writing Committee for the Diabetic Retinopathy Clinical Research N, Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG et al. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol 2007; 125 (4): 469–480.
Escobar-Barranco JJ, Pina-Marin B, Fernandez-Bonet M . Dexamethasone implants in patients with naive or refractory diffuse diabetic macular edema. Ophthalmologica 2015; 233 (3-4): 176–185.
Jimenez-Gomez B, Gonzalez-Montpetit M, Fonollosa Calduch A, Orive Banuelos A, Valsero Franco S . Effects of ozurdex on intraocular pressure. A real life clinical practice study. Arch Soc Esp Oftalmol 2015; 90 (9): 421–425.
Thakur A, Kadam R, Kompella UB . Trabecular meshwork and lens partitioning of corticosteroids: implications for elevated intraocular pressure and cataracts. Arch Ophthalmol 2011; 129 (7): 914–920.
Meyer LM, Schonfeld CL . Secondary glaucoma after intravitreal dexamethasone 0.7 mg implant in patients with retinal vein occlusion: a one-year follow-up. J Ocul Pharmacol Ther 2013; 29 (6): 560–565.
Hayreh SS, Zimmerman MB, Beri M, Podhajsky P . Intraocular pressure abnormalities associated with central and hemicentral retinal vein occlusion. Ophthalmology 2004; 111 (1): 133–141.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chhablani, J., Bansal, P., Veritti, D. et al. Dexamethasone implant in diabetic macular edema in real-life situations. Eye 30, 426–430 (2016). https://doi.org/10.1038/eye.2015.246
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2015.246
This article is cited by
-
Differential response to intravitreal dexamethasone implant in naïve and previously treated diabetic macular edema eyes
BMC Ophthalmology (2020)
-
Intravitreal dexamethasone implant as an alternative to systemic steroids as prophylaxis for uveitic cataract surgery: a randomized trial
Eye (2020)
-
Baseline SD-OCT characteristics of diabetic macular oedema patterns can predict morphological features and timing of recurrence in patients treated with dexamethasone intravitreal implants
Acta Diabetologica (2020)
-
Intravitreal dexamethasone implant Ozurdex® in naïve and refractory patients with different subtypes of diabetic macular edema
BMC Ophthalmology (2019)
-
Dexamethasone Intravitreal Implant in Diabetic Macular Edema: Real-Life Data from a Prospective Study and Predictive Factors for Visual Outcome
Diabetes Therapy (2017)