Abstract
Purpose
To compare 2.0 mg ranibizumab (RBZ) injections with 0.5 mg RBZ for eyes with center-involved diabetic macular edema (DME) and a central subfield thickness (CFT) of ≥250 μm on time-domain optical coherence tomography.
Design
Randomized, controlled, multicenter clinical trial.
Methods
Eligible eyes were randomized in a 1:1 ratio to 0.5 mg (n=77) or 2.0 mg (n=75) RBZ. Study eyes received 6-monthly injections.
Main outcome measures
The primary outcome measure was the mean change in best corrected visual acuity (BCVA) at month 6. Secondary outcomes included the incidence and severity of systemic and ocular adverse events and the mean change in CFT from baseline.
Results
In all, 152 eyes (152 patients) were randomized in the study. At month 6, the mean improvement from baseline BCVA was +9.43 letters in the 0.5 mg RBZ group and +7.01 letters in the 2.0 mg RBZ group (P=0.161). At month 6, one death occurred in the 0.5 mg RBZ group and three deaths in the 2.0 mg RBZ group, all due to myocardial infarction in subjects with a prior history of heart disease. Mean CFT was reduced by 168.58 μm in the 0.5 mg RBZ group and by 159.70 μm in the 2.0 mg RBZ group (P=0.708).
Conclusions
There was no statistically significant difference in the mean number of letters gained between the 0.5 and 2.0 mg RBZ groups through month 6. In this DME study population, high-dose RBZ does not appear to provide additional benefit over 0.5 mg RBZ.
Similar content being viewed by others
Introduction
Vascular endothelial growth factor (VEGF) plays an important role in the pathogenesis of diabetic macular edema (DME).1 Studies have shown that VEGF levels are elevated in the retina and vitreous of eyes with diabetic retinopathy. Randomized clinical trials, such as the Ranibizumab for Edema of the mAcula in Diabetes-2 (READ-2) Study, have demonstrated that treatment with an intravitreal VEGF inhibitor (ranibizumab, RBZ) results in superior visual acuity outcomes compared with focal/grid laser or triamcinolone.2, 3, 4, 5, 6, 7 In fact, intravitreal VEGF inhibitors have become the new standard of care for the treatment of center-involved DME, and 0.3 mg RBZ has been approved by the United States Food and Drug Administration (FDA) for DME.
Based on the available data from DME studies with RBZ, repeated intravitreal injections are required to maintain disease stability and to induce improvement in visual acuity.4, 6, 7 The Studies of Ranibizumab Injection in Subjects with Clinically Significant Macular Edema (ME) With Center Involvement Secondary to Diabetes Mellitus (RIDE and RISE) showed that monthly dosing with both 0.3 and 0.5 mg RBZ resulted in greater visual acuity outcomes compared with sham treatment.6 In addition, the Diabetic Retinopathy Clinical Research Network demonstrated that a modified dosing regimen based on the presence of center-involved DME with a median of 8 to 9 RBZ injections over the first 12 months resulted in a gain of approximately 9 letters of visual acuity.4 Alternative approaches to the current treatment regimen may be useful to reduce the burden of follow-up and treatments to patients and retina specialists. It is important to determine if higher doses of a VEGF antagonist such as RBZ are safe and can improve bioactivity as measured by changes in visual acuity and/or retinal thickness, or can reduce the frequency of treatments in eyes with DME.
The READ-3 Study is a double-blind randomized multi-center clinical trial comparing 0.5 and 2.0 mg RBZ for the treatment of center-involved DME.
Materials and methods
The READ-3 Study is a randomized clinical trial conducted at 13 sites in the United States through an investigator-initiated investigational new drug application granted by the FDA. The names of investigators, coordinators, and staff members from all sites that participated in the READ-3 Study are listed at the end of the paper. The study adheres to the guidelines of the Declaration of Helsinki and HIPAA, and the protocol and consent form are approved by a local institutional review board (IRB) for selected sites and by Western IRB for others. Each subject provided written informed consent. The study is monitored by an independent Data and Safety Monitoring Committee (DSMC) that monitored adverse events and data at regular intervals. The study is registered at www.clinicaltrials.gov under the identifier NCT01077401.
Patient eligibility and exclusion criteria
Patients (18 years or older) with type 1 or type 2 diabetes and DME were eligible if they had reduction in visual acuity between 20/40 and 20/320 and met the following criteria: (1) central subfield (CSF) thickness measured by time-domain optical coherence tomography (TD-OCT)≥250 μm, (2) HbA1c≥6% within 12 months prior to randomization, (3) no other confounding ocular condition that could decrease visual acuity aside from DME, and (4) reasonable expectation that scatter laser photocoagulation would not be required for the next 6 months. Patients were excluded if they had received focal/grid laser treatment within 3 months, intraocular injection of steroid within 3 months, or intraocular injection of a VEGF antagonist within 2 months. If both eyes were eligible, the eye with the greater CSF thickness was enrolled.
Study protocol
Consenting patients were screened for the study with a medical history, measurement of best corrected visual acuity (BCVA) by a masked, certified visual acuity examiner using the Early Treatment Diabetic Retinopathy (ETDRS) protocol, slit-lamp examination, measurement of intraocular pressure, dilated funduscopic examination, time-domain and spectral-domain OCT (SD-OCT), fluorescein angiography (FA), and laboratory tests. Eligible patients were randomized 1:1 (in blocks of 4 using the built-in randomization tool of the EDC) to injections of 0.5 mg of RBZ or 2.0 mg of RBZ. The dose of 0.5 mg RBZ was used in this trial because 0.3 mg RBZ was not yet FDA approved for DME at the time of enrollment. Although the 0.3 mg dose gained FDA approval during the study period, the DSMC elected to continue using the 0.5 mg dose for the entire clinical trial duration because both doses are efficacious and there are no clear safety signals associated with the latter dose. Patients in both groups received an injection of RBZ at baseline and months 1, 2, 3, 4, and 5. Month 6 was the primary end point of the study. After month 6, patients in both groups were evaluated every month and were eligible to receive additional RBZ if the CSF thickness was ≥250 μm on TD-OCT or if there was evidence of any macular fluid on either TD-OCT or SD-OCT. Safety evaluations, measurement of BCVA, eye examinations, and OCTs were done at all study visits. FA and HbA1c measurements were performed at baseline and month 6. The procedures for intravitreal injections, OCT, and data collection and management have been previously described.2, 5
Sample size calculation
The sample size was selected to allow the study to have an 80% power to detect a difference of 4 or more ETDRS letters between the two arms. This size makes the READ-3 study comparable to other clinical trials, as 80% is the typical power employed in many major clinical trials.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
Patient disposition and treatment
A total of 152 eyes were randomized in the study. The baseline characteristics were fairly balanced among the two study groups apart from female gender and African American race, both of which had a higher percentage in the 2.0 mg dose group (Table 1); the mean BCVA was 26.30 letters (20/80 Snellen equivalent) in the 0.5 mg RBZ group and 29.25 letters (20/63 Snellen equivalent) in the 2.0 mg RBZ group. In addition, the baseline CSF thicknesses were 441.37 and 432.33 μm in the 0.5 and 2.0 mg groups, respectively. Ninety-three (93) percent of subjects completed the month-6 study visit. Six patients in the 2.0 mg RBZ group and four patients in the 0.5 mg RBZ group did not complete the month-6 visit for the following reasons: five patients left on their own choice, four patients passed away, and one patient left at the investigator’s recommendation.
The median numbers of RBZ injections during the first 6 months were 5.66 and 5.60 (out of 6 mandatory) in the 0.5 and 2.0 mg RBZ groups, respectively. In all, 45.3% of patients were treatment naive in the 2.0 mg group, whereas 53.2% of patients were treatment naive in the 0.5 mg group. Previous treatments have been listed in Table 2.
Visual outcomes
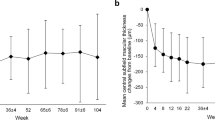
At month 6, the primary end point of the study, the mean improvement from baseline in BCVA in ETDRS letter score, was +9.34 in the 0.5 mg RBZ group and +7.04 in the 2.0 mg RBZ group (P=0.161). The mean difference between the two groups was 2.30 letters and the confidence interval was 0.8–5.48. Figure 1a illustrates the mean improvement from baseline in BCVA at different time points in the study for the two groups.
Mean change from baseline in best corrected visual acuity and central subfield thickness. (a) Mean change from baseline in best corrected visual acuity (BCVA): changes in mean BCVA from baseline, as measured by the number of Early Treatment Diabetic Retinopathy Study (ETDRS) letters read at different time points during the study for each treatment group. (b) Mean change from baseline in central subfield (CSF) thickness: changes in mean CSFthickness from baseline, at different time points during the study for each treatment group.
Twenty-six eyes improved by ≥10 letters, and 18 eyes improved by≥15 letters in the 0.5 mg RBZ group. Twenty-nine eyes improved by≥10 letters, and 8 eyes improved by ≥15 letters in the 2.0 mg RBZ group. Table 3 illustrates the number of patients that either gained or lost ≥10 and ≥15 letters in the two groups.
Treatment naive versus previously treated eyes
In the 2.0 mg RBZ group, at month 6, the mean improvement from baseline in BCVA in ETDRS letter score was 8.84 letters in treatment-naive eyes compared with 5.49 letters in previously treated eyes (anti-VEGF agents, corticosteroids, or laser). Similarly, in the 0.5 mg RBZ group, the mean improvement in BCVA in ETDRS letter score was 10.09 letters in treatment-naive eyes compared with 8.77 letters in previously treated eyes.
Anatomic outcomes
The mean CSF thickness in the 0.5 mg RBZ group decreased by 168.58 μm compared with a mean decrease of 159.70 μm in the 2.0 mg RBZ group at month 6 (P=0.708). Figure 1b illustrates the mean change from baseline in CSF thickness in the two study groups at different time points during the study.
Treatment-naive versus previously treated eyes
In the 2.0 mg RBZ group, at month 6, the mean decrease in CSF thickness from baseline was 183.80 μm in treatment-naive eyes compared with 154.2 μm in previously treated eyes. Similarly, in the 0.5 mg RBZ group, the mean decrease in CSF thickness from baseline was 182.5 μm in treatment-naive eyes compared with 166.8 μm in previously treated eyes.
Safety
Among the study participants, one subject in the 0.5 mg RBZ group died from myocardial infarction and three subjects in the 2.0 mg RBZ group died from myocardial infarction (Table 4a). All four study subjects who died had a prior history of coronary artery disease. There were no cerebrovascular events in either group. Worsening hypertension was diagnosed in two subjects in the 0.5 mg RBZ group and in no subjects in the 2.0 mg RBZ group. One patient in the 2.0 mg RBZ group developed a deep venous thrombosis.
In regards to serious ocular adverse events in the study eye, there were no cases of endophthalmitis or ocular inflammation in either treatment group (Table 4b). The number of eyes that lost 30 or more letters was 1 in the 0.5 mg group and 0 in the 2.0 mg group. The case in the 0.5 mg group was attributed to an increase in macular edema by the study investigator.
Discussion
The READ-3 Study was designed to evaluate the effects of 0.5 and 2.0 mg RBZ on visual acuity for center-involved DME through 6 months of treatment. At the time of design of the READ-3 Study, RBZ (of any dose) had not been approved by the FDA for DME; therefore, the comparison between 0.5 and 2.0 mg RBZ was selected for this trial. After six scheduled monthly injections from baseline to the primary end point of month 6, the mean difference between the two treatment groups was 2.30 letters (favoring 0.5 mg RBZ) and the confidence interval was 0.8–5.48. Therefore we can assume with 95% confidence that the mean difference between the two arms lies between the given intervals. There was no evidence to suggest that the difference in letter gain between the two treatment arms is more than six letters. In this small study population, the 2 mg dose of RBZ did not appear to provide any additional visual acuity benefits compared with the 0.5 mg dose. In addition, changes in CSF thickness on OCT also did not show any advantage for the 2.0 mg dose of RBZ.
A higher number of deaths due to myocardial infarction were reported in the 2.0 mg RBZ group compared with the 0.5 mg dose. These deaths occurred in subjects who had a prior history of coronary artery disease and were at higher risk for arteriothrombotic events. There were no cases of cerebrovascular accidents among the READ-3 study participants through 6 months. In regards to hypertension, which is a known adverse event associated with systemic VEGF inhibitors, there were two cases of worsening hypertension in the 0.5 mg group and no cases in the 2 mg group. One would expect to see more cases of hypertension among the subjects who received 2 mg of RBZ, but this was not seen in our study population. The slightly elevated death rate seen in the READ-3 study was also seen in the RISE and RIDE studies between the 0.3 and 0.5 mg RBZ doses, with the 0.5 mg dose group being associated with more serious systemic adverse events;6 however, these phase III studies were not powered to adequately evaluate this safety question. Similarly, the READ-3 study was not powered to detect a small difference in uncommon systemic adverse events because it would require an extremely large patient population to answer safety questions; therefore, no conclusion on the risk for higher rates of serious systemic adverse events with higher doses of intravitreal VEGF inhibition can be made with any of the current clinical trial data. Additional large-scale studies with proper pre-determined power involving tens of thousands of participants are necessary to truly answer this safety question.
The data from the READ-3 study may have important clinical implications. First, it is the largest randomized clinical trial to date to evaluate a 2.0 mg dose of RBZ for DME. Although diabetic retinopathy is associated with retinal hypoxia and upregulation of intraocular VEGF, the fact that there was no additional visual acuity benefit or further reduction in CSF thickness in eyes treated with the higher dose of RBZ suggests that the 0.5 mg dose is at the top of the dose–response curve and already provides the maximum amount of VEGF inhibition. Although the study reported a trend for better visual acuity gains in eyes receiving the lower dose, a significant difference in the visual outcomes between the two groups was not seen.
Possible limitations of the READ-3 Study include selection bias in eyes that were previously treated, as investigators may have included more patients with chronic and unresponsive eyes in a study that offered treatment with a higher dose of RBZ. Another possible limitation of the study was that a higher percentage (53.2 vs 45.3) of patients in the low-dose group were treatment naive. The patients in the low-dose group also had a lower baseline VA. These factors may have been responsible for the slightly better visual gains seen in the low-dose group. A larger study or longer follow-up and additional treatment may show differences in visual acuity benefits among the two doses of RBZ. Interestingly, the HARBOR study, comparing the 0.5 and 2.0 mg doses of RBZ for the treatment of neovascular age-related macular degeneration, also demonstrated that this higher dose of RBZ did not result in greater visual acuity benefits compared with the 0.5 mg dose. At this time, the READ-3 data suggest that increasing the concentration of RBZ during a single injection does not provide additional benefit for their DME patients.

References
Nguyen QD, Tatlipinar S, Shah SM, Haller JA, Quinlan E, Sung J et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol 2006; 142 (6): 961–969.
Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D et al. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2009; 116 (11): 2175–81 e1.
Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2010; 117 (11): 2146–2151.
Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010; 117 (6): 1064–1077 e35.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011; 118 (4): 615–625.
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012; 119 (4): 789–801.
Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Sophie R et al. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol 2013; 131 (2): 139–145.
Acknowledgements
READ-3 was an investigator-sponsored study with the IND (investigative new drug) for the Study held by one of the investigators (QDN). The READ-3 Study was funded by JDRF International with the study drug provided by Genentech, Inc.QDN has received grant support at his institution from Genentech and Regeneron and is a consultant with Santen. DB has received grant support, travel support, and is a consultant with Genentech. He is also a member of the scientific advisory board at Genentech. He is a consultant with Aerpio, Alcon, Allegro, Allergan, Bausch & Lomb, Bayer, GSK, KalVista, Neurotech, Nicox, Novartis, Ohr, Regeneron, Santaris, Santen, and Thrombogenics. He also receives payment for lectures from Alcon and Allergan. He is a stockholder with Allegro, Neurotech, and Ohr. He sits at the scientific advisory board of Alcon, Allergan, Novartis, Neurotech, Pfizer, and Stem Cells, Inc. DC has received grant support at his institution from JDRF. He is a consultant to Alcon, Allergan, and Regeneron; and receives payment for lectures from Allergan. He is a stockholder with ForSight. DM is a consultant with Regeneron and Genentech. LH is a consultant with and has received travel support from Regeneron and Alcon.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
QDN is a recipient of a Physician Scientist Award from Research to Prevent Blindness, New York, NY. PAC is the George S. and Dolores Doré Eccles Professor of Ophthalmology and Neuroscience. QDN is a consultant for Bausch and Lomb and Santen, and has research support from Genentech, MacuSight, Santen, L-Path, Ophthotech, and Regeneron, and has institutional consulting agreement with AbbVie, and honorarium from Bayer, XOMA, Heidelberg, and Quantel. QDN also chairs the Steering Committee for the RISE and RIDE Study, and is on the Steering Committee for the VISTA Study, and other studies sponsored by Genentech and Regeneron. DVD is a consultant for Genentech, Regeneron, Santen, and Allergan and she has received research support from Genentech and Regeneron, and honorarium from Heidelberg and Quantel. DVD also chairs the Steering Committee for the VISTA Study. PAC has institutional consulting agreements with Genentech and GlaxoSmithKline, is a consultant for Allergan, and formerly consulted for Amira, Potentia, and LPath, and served on the data and safety monitoring committee for a phase III trial sponsored by Regeneron, Inc., and has research support from Genentech, Alimera, and Molecular Partners for diabetic macular edema trials and GlaxoSmithKline, Genezyme, and Oxford BioMedica for neovascular AMD trials. These activities are being managed by the Conflict of Interest Committee of the Johns Hopkins University School of Medicine. LH is a stockholder in Covalent Medical LLC and a consultant to Regeneron Pharmaceuticals. DB is a consultant for Allergan, Alcon, Bausch and Lomb, Genentech, Regeneron, Allegro, Neurotech, Ohr, Quantel, Thrombogenics, Allergan, Alcon, Ampio, Thrombogenics, Allegro, Novartis, Roche, and Pfizer. DC is a consultant for Allergan, Alcon, Bausch & Lomb, and Regeneron. DMM is an advisory board member and consultant for Genentech, Regeneron, and Thrombogenics, and receives clinical research support from Genentech, Regeneron and Thrombogenics, Alcon, Allergan, GSK, Pfizer, Acucela, Lpath, Ophthotech, and Quark. The remaining authors declare no conflict of interest.
Additional information
This study was presented in part at the American Academy of Ophthalmology Retina Subspecialty Day, 2012.
Appendix
Appendix
Appendix Investigators, Coordinators, and Staff Members of the READ-3 Study
A. Clinical Sites
1. Wilmer Eye Institute, Johns Hopkins University (Baltimore, MD). PI: Diana V. Do, M.D. Study Coordinator: Jennifer Belz
2. Southeast Retina Center (Augusta, GA). PI: Dennis Marcus, M.D. Study Coordinator: Allison Foster
3. East Bay Retina Institute (Oakland, CA). PI: Eugene Lit, M.D. Study Coordinator: Scotty Renslow
4. Eye Care Specialists (Kingston, PA). PI: Erik Kruger, M.D. Study Coordinator: Patty Yuhas
5. Illinois Retina Associates (Chicago, IL). PI: John Pollack, M.D. Study Coordinator: Barbara Ciscato
6. Retina Group of Florida (Fort Lauderdale, FL). PI: Larry Halperin, M.D. Study Coordinator: Jackie Lopez
7. Retina Institute of Hawaii (Honolulu, HI). PI: Michael Bennett, M.D. Study Coordinator: K'Marie Rego
8. Retina Macula Institute (Torrance, CA). PI: Ron Gallemore, M.D. Study Coordinator: Lillian Chen
9. Retina Vitreous Associates (Beverly Hills, CA). PI: David Boyer, M.D. Study Coordinator: Amanda Tam
10. Texas Retina Associates (Arlington, TX). PI: David Callanan, M.D. Study Coordinator: Sandy Lash
11. Shiley Eye Center, University of California, San Diego (San Diego, CA). PI: Kang Zhang, M.D., Ph.D. Study Coordinator: Maureen Crocker
12. University of Kansas (Kansas City, KS). PI: Andrew Symons, M.D. Study Coordinator: Rebecca Bothwell
13. Black Hills Eye Institute (Rapid City, SD). PI: Prema Abraham, M.D. Study Coordinator: Buffi Green
B. Executive Committee of the READ-3 Study
David Boyer, MD
David G Callanan, MD
Peter A Campochiaro, MD
Quan Dong Nguyen, MD, MSc, Chair
C. Data Safety and Monitoring Committee
Brian P Conway, MD
David J Wilson, MD
D. Reading Center
The Ocular Imaging Research and Reading Center at the Stanley M Truhlsen Eye Institute, University of Nebraska Medical Center (Omaha, NE)
asir Jamal Sepah, MBBS
Mohammad Ali Sadiq, MD
Mostafa Hanout, MD
E. Data Collection and Monitoring Center
The Ocular Imaging Research and Reading Center at the Stanley M. Truhlsen Eye Institute, University of Nebraska Medical Center (Omaha, NE)
Salman Sarwar, MD
Jose Maya, MD
Nithya Rajagopalan
Aniruddha Agarwal, MD
F. Coordinating Center
Stanley M Truhlsen Eye Institute, University of Nebraska Medical Center (Omaha, NE)
Mostafa Hanout, MD
Mohamed K Soliman, MD
Lisa Greer, MBA
G. Statistical Analyses
Mohammad Ali Sadiq, MD
Yasir Jamal Sepah, MBBS
Rights and permissions
About this article
Cite this article
Do, D., Sepah, Y., Boyer, D. et al. Month-6 primary outcomes of the READ-3 study (Ranibizumab for Edema of the mAcula in Diabetes—Protocol 3 with high dose). Eye 29, 1538–1544 (2015). https://doi.org/10.1038/eye.2015.142
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2015.142
This article is cited by
-
Differences in the characteristics of subjects achieving complete, partial, or no resolution of macular edema in the READ-3 study
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
A Review of Ranibizumab for the Treatment of Diabetic Retinopathy
Ophthalmology and Therapy (2017)
-
Aflibercept in diabetic macular edema: evaluating efficacy as a primary and secondary therapeutic option
Eye (2016)
-
Diabetic macular oedema: pathophysiology, management challenges and treatment resistance
Diabetologia (2016)
-
Novel Therapies in Development for Diabetic Macular Edema
Current Diabetes Reports (2015)