Abstract
Purpose
To evaluate the refractive outcomes in children treated after intravitreal injection of bevacizumab (IVB) for retinopathy of prematurity (ROP).
Methods
A retrospective, bi-centre study of 34 patients (64 eyes) was conducted. The patients were divided into three groups, patients received intravitreal IVB (IVB group), patients received combined IVB and laser treatment (IVB+Laser group), or patients received lens-sparing vitrectomy (IVB+LSV group). Cycloplegic refraction and axial length (AXL) were evaluated at 2 years old.
Results
The prevalences of myopia and high myopia were 47.5 and 10.0% in the IVB group, respectively, which were lower than those in the IVB+Laser (82.4 and 29.4%) and IVB+LSV (all 100%) groups (P=0.001 and P<0.001). The prevalences of emmetropia in the IVB group, IVB+Laser group, and IVB+LSV group were 50, 5.9, and 0% (P=0.001). The AXL were similar among all groups.
Conclusions
At the 2-year follow-up, severe ROP patients treated with IVB alone were more likely to remain emmetropic and had lower prevalences of myopia and high myopia. The development of high myopia in severe ROP patients could not be explained by AXL changes but may be associated with abnormalities in the anterior segment.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative retinopathy that involves the developing retinas of premature infants. ROP is a cause of major visual morbidity in children and is responsible for up to 15% of all cases of blindness in developed countries and up to 60% of cases in middle-income countries.1
Since the 1980s, ablation using cryotherapy and, subsequently, laser photocoagulation of the peripheral avascular retina have been considered to be the gold standards to reduce the cicatricial sequelae of ROP.2 However, many investigators have reported high prevalences of myopia and high myopia in preschool- and school-age children who received ablative therapy.3, 4, 5, 6, 7 For these patients, it has been reported that laser treatment for severe ROP results in less myopia than cryotherapy.6
In the last several years, anti-vascular endothelial growth factor (VEGF) agents have emerged as a new treatment for ROP, and promising experiences have been reported.8, 9, 10 However, only a few case series have reported the refractive outcomes after the injection of an anti-VEGF agent in these ROP patients.9, 10, 11, 12 These reports showed lower prevalences of myopia and high myopia in anti-VEGF-treated patients than in laser-treated patients.9, 10, 11, 12 The refractive outcomes after anti-VEGF treatment for ROP and in combination with laser or vitrectomy have not been reported. The aim of the current study aims to evaluate the refractive changes in preterm infants with type 1 ROP 2 years after treatment with intravitreally injected bevacizumab (Avastin; Genentech Inc., San Francisco, CA, USA) (IVB), an anti-VEGF antibody. Three groups of ROP patients after IVB were analysed: the patients received IVB only (IVB group), patients received combined IVB and laser treatment (IVB+Laser group), or patients received lens-sparing vitrectomy (LSV) (IVB+LSV group).
Subjects and methods
From August 2010 to November 2011, a retrospective study was performed at two hospitals, the Linkou branch of Chang Gung Memorial Hospital, Taoyuan, Taiwan and Chang-Hua Christian Hospital, Chang-Hua, Taiwan. Study protocols were approved by the review boards of the two participating institutions and the procedures used conformed to the tenets of the Declaration of Helsinki.
The infants were screened for prematurity if their birth weight (BW) was <1500 grams or their gestational age (GA) <32 weeks. Infants who did not meet these criteria but exhibited an unstable clinical course and required cardiorespiratory support were also screened. The first screening time point was at a postnatal age of 4–6 weeks, and follow-up examinations were performed according to the guidelines published by the American Academy of Ophthalmology, American Academy of Pediatrics and American Association for Pediatric Ophthalmology and Strabismus.13 The findings were classified according to the International Classification of ROP criteria.14
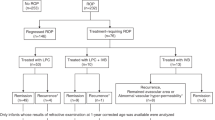
Infants who developed type 1 ROP defined by the Early Treatment for ROP(ET-ROP) study were provided the option of IVB or laser treatment after a thorough discussion with their parents.15 The off-label use of IVB as an alternative treatment to laser treatment was explained to the parents. If the parents chose to treat their child with IVB, a dose of 0.625 mg (0.025 ml) bevacizumab was injected via the pars plicata under intravenous sedation. If the ROP recurred or if there was a lack of treatment response following IVB, additional laser treatment to stop the progression of ROP was offered. If the ROP failed to respond to laser treatment, off-label use of IVB was used. Infants who progressed to retinal detachment without macular involvement (stage 4A ROP) were treated with (LSV).
At 2 years of age, the patients were included in the study if their ROP had regressed after treatment, their retinas were found to be attached during the follow-up, and they were cooperative during the refraction check-up. Patients who were not able to undergo the examinations, who developed any form of cataracts or received cataract surgery, who developed cornea opacity, who had stage 4B ROP or worse, or who developed glaucoma were excluded.
Infants were evaluated for refractive errors and axial length (AXL) at the age of 2. The infants’ eyes were assessed to identify refractive errors 30 min after instilling 2% cyclopentolate and 1% tropicamide (two instillations with an interval of 10 min). A handheld auto-refractometer (FR-5000; Grand Seiko Co., Ltd., Hiroshima, Japan) was used to evaluate the cycloplegic refraction. The results were recorded in the form of spherical, cylinder, astigmatism degree and axis, type of astigmatism, and spherical equivalent (SE, spherical plus half of the cylinder power). At least three measurements were taken to obtain an average reading. Myopia was defined as SE ≤−0.25 D, and high myopia was defined as SE ≤−5.00 D.5 High astigmatism was defined as minus cylinder form >1.50 D. Emmetropia was defined as SE >−0.25 D but <2 D. For the analysis of the axis of astigmatism, three classes were recorded—‘with the rule’ (WTR): cylinder at 0° to 30° or 150° to 180°; ‘against the rule’ (ATR): cylinder at 60° to 120°; and ‘oblique type’: cylinder at 31° to 59° or 121° to 149°.16 Measurements of the AXL were obtained with an optical coherence biometer (IOL Master; Carl Zeiss, Jena, Germany). The patients were divided into those who received IVB only and whose ROP responded well to this treatment (IVB group), those who received both IVB and laser treatment due to the recurrence of ROP or a lack of response to a single treatment (IVB+Laser group), and those who also received a vitrectomy for retinal detachment associated with ROP (IVB+LSV group).
The statistical analysis was performed using SPSS 17 for Windows 7 (SPSS, Inc., Chicago, IL, USA). For the comparison of the three groups, the numerical data were analysed using the Kruskal–Wallis test, and the categorical data were analysed using Fisher’s exact test. Two-tailed P-values of <0.05 were considered to be statistically significant.
Results
Patient characteristics
At the two clinical sites, 45 patients with type 1 ROP history presented for ocular examinations at 2 years old. Eight eyes of five patients developed cataracts and were treated with surgery; three eyes of three patients were subjected to vitrectomy-assisted lensectomy; and four eyes of three patients had stage 4B or five ROP. These eyes were excluded from the present study. None of the eyes developed corneal opacity or glaucoma. In total, 34 patients (20 boys and 14 girls; 64 eyes) met the study’s inclusion criteria and were enrolled. The final data analysis was performed for 40 eyes that received IVB only (IVB group), 17 eyes that had combined IVB and laser ablative treatment (IVB+Laser group), and 7 eyes that had advanced to stage 4A ROP and were treated with a vitrectomy (IVB+LSV group).
The mean GA of the patients was 26.4±2.3 weeks (range, 23 to 32 weeks). The mean BW of the patients was 882.2±265 grams (range, 520 to 1625 grams). Five eyes (7.8%) developed stage 2 ROP, 52 eyes (81.3%) developed stage 3 ROP, and 7 eyes (10.9%) developed stage 4A ROP. There were 11 eyes (17.2%) that developed zone 1 ROP. The mean postmenstrual age (PMA) at the time of first treatment with IVB was 35.0±2.8 weeks (range, 31.2 to 44.1 weeks). The mean GA was significantly different among the groups (P=0.012), but the mean BW was similar among the groups (P=0.103). The demographic information for all patients was presented in Table 1.
Refraction outcomes at 2 years
The average refractive errors of patients at 2 years old in the IVB, IVB+Laser, and IVB+LSV groups were −0.98±4.05 D (range, −15.6 to 5.5 D), −2.40±3.13 D (range, −7.6 to 2.9 D), and −14.38±6.02 D (−21.8 to −8.1 D), respectively (P<0.001) (Table 2). Myopia (SE ≤−0.25 D) was observed in 19 eyes (47.5%) in the IVB group, 14 eyes (82.4%) in the IVB+Laser group, and 7 eyes (100%) in the IVB+LSV group (P=0.001). Four eyes (10%) in the IVB group, five eyes (29.4%) in the IVB+Laser group, and seven eyes (100%) in the IVB+LSV group were discovered to have high myopia (SE ≤−5.00 D) (P<0.001) (Table 2). Fewer patients in the IVB group developed myopia and high myopia. The prevalence of emmetropia (SE >−0.25 D but <2 D) was also the highest in the IVB group (P=0.001). Twenty eyes (50%) in the IVB group, one eye (5.9%) in the IVB+Laser group, and none of the eyes in the IVB+LSV group developed emmetropia (Table 2).
The average levels of astigmatism of these patients at 2 years old were similar: 2.23±1.53 D, 2.32±1.10 D, and 3.11±1.54 D in the IVB, IVB+Laser, and IVB+LSV groups, respectively. There were also no significant differences between the percentages of high astigmatism (>1.5 D) in the different groups (Table 2). Astigmatism was further categorised into the WTR, ATR, and oblique types for further analysis. In IVB patients, 34 eyes (85%) developed WTR astigmatism. Sixteen eyes (94.1%) in the IVB+Laser group and six eyes (85.7%) in the IVB+LSV group also developed WTR astigmatism. These results are shown in Table 2. Most patients developed WTR astigmatism, and no significant differences among groups were discovered.
Table 2 shows the AXL measurements at 2 years old. The average measurements for the AXL were 21.30±0.78 mm (range, 19.76 to 23.10 mm), 21.44±1.44 mm (range, 19.25 to 24.68 mm), and 21.85±1.52 mm (range, 20.35 to 23.77 mm) for the IVB, IVB+Laser, and IVB+LSV groups, respectively. There were no differences found among the groups.
Discussion
We found lower prevalences of myopia and high myopia at 2 years in the IVB group than in the IVB+Laser and IVB+LSV groups (P=0.001 and P<0.001). The prevalence of emmetropia was also higher among the patients who were treated with IVB only (P=0.001). The prevalence of astigmatism was similar among groups, and most of the study eyes had WTR astigmatism. The AXL measurements at 2 years old were similar. Currently, there are only three case series and one case report that discuss the refractive changes after the use of IVB.9, 10, 12, 17 To the best of our knowledge, this is the largest study cohort to examine refraction composition in infants who received IVB-based treatment. Previous studies only reported the refractive outcomes after IVB or the non-comparative outcomes after IVB or laser treatment.9, 10, 12, 17
Many authors have stated that myopia occurs frequently in infants who develop ROP and increases with the severity of the ROP.5, 6 The refractive error abnormalities of ROP patients have been found to present early in infancy and persist into adulthood.16 However, myopia and high myopia occurred more frequently after pre-threshold or threshold ROP patients were treated with peripheral laser therapy. The reported rates range from 55.2 to 80.4% and 23.9 to 31.5%, respectively (Table 3).3, 4, 6 The reported rates of myopia and high myopia were even higher in patients treated with cryotherapy, ranging from 82.9 to 92.0% and 32.0 to 52.5%, respectively (Table 3).5, 6, 7 In the current study, the percentages of patients with myopia and high myopia among IVB-treated patients were 47.5 and 10.0%, respectively, which are less than the previously reported percentages for patients treated with laser treatment or cryotherapy.
Few investigators have reported the refractive outcomes after the use of IVB (Table 3). The reported mean SE was between −1.04 to 0.64 D between 1 and 2 years old, which was similar to that of our patients who were treated with IVB only (−0.98 D).9, 10, 17 Our study and other case series found a lower SE in patients treated with IVB than in patients treated with cryotherapy (range, −6.5 to −3.51 D) or with laser peripheral ablative therapy (range, −4.71 to −1.50 D).3, 4, 5, 6, 7 Our prevalences of myopia and high myopia are also similar to those of preschool-age patients in a recent case series who were treated with IVB only (55.6% and 11.1 to 17.0%) (Table 3).9, 10, 17
In the current study, the prevalences of myopia and high myopia increased significantly with the severity of ROP but were not associated with AXL changes. It is commonly understood that eyes treated with cryotherapy and peripheral laser photocoagulation are more myopic. However, the role of these treatments in the development of myopia is controversial. Many investigators have reported that cryotherapy and laser ablation may not increase the risk of myopia after controlling for the severity of ROP.5, 7
Some studies have also found similar AXL measurements in patients with different severities of ROP.18, 19 It is now recognised that ROP and prematurity-induced myopia cannot be fully explained by increased AXL and that anterior segment arrest contributes to the increased myopia observed in these conditions. Many investigators have reported that ROP eyes have significantly steeper corneal curvatures, shallower anterior chamber depths, greater lens thicknesses, and increased macular thicknesses.18, 19 Our results further indicate that the high refractive errors in patients with additional laser treatment or LSV were not due to an increase in the AXL. It is most likely that the refractive errors of these patients are due to abnormalities in the anterior segment. Unfortunately, our study design did not include other biometric measures such as cornea curvature, anterior chamber depth, or lens thickness because these measurements require the cooperation of these young children.
Several theories have been suggested to explain the biometric changes observed in myopia of prematurity after LSV. Vitrectomy surgery in ROP patients may compromise the ocular perfusion pressure, leading to retinal and choroidal ischaemia and further damage to the retina and optic nerve.20 Holz reported that eyes subjected to LSV may exhibit disrupted emmetropisation due to trophic factor changes.21 If these factors are involved with the development of cornea collagen or lens protein, LSV could induce refractive errors in the eyes by changing the levels of these trophic factors. Further study is still needed to explore the cause of high refractive errors after LSV in these ROP patients.
Previous studies found high astigmatism with divergent axes in children with a history of ROP.18, 22 Holmström observed that the ATR type of astigmatism was more common in ROP patients.22 Another study of a cohort of subjects between 7 and 9 years old found that of the 44% of patients with ROP among 108 children with a history of prematurity, 70% had WTR astigmatism.18 In the present study, most children developed WTR astigmatism. Further study will be required to determine the reasons for this difference. A new finding of our study is that the majority of patients who received IVB treatment developed WTR astigmatism, a result that has not been previously reported in the literature.
Our study is limited by small number of patients enrolled, the retrospective study design, no retinoscopy performed in the patients, none of the patients who received laser treatment as mono-therapy returned to clinics for the 2-year-old follow-up, and therefore there was a lack of biometric measurements. Despite these limitations, this study may still provide useful information on ROP patients who were treated with IVB-based therapy.
In conclusion, our bi-centre study confirmed that there are lower prevalences of myopia and high myopia and a higher prevalence of emmetropia in children with type 1 ROP who were treated with IVB. Most of the patients developed WTR astigmatism. The development of high myopia in severe ROP patients was not due to AXL changes and was most likely related to abnormalities in the corneal curvature, anterior chamber depth, and lens thickness.

References
Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics 2005; 115: e518–e525.
Early treatment for retinopathy of prematurity cooperative group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003; 121: 1684–1694.
Axer-Siegel R, Maharshak I, Snir M, Friling R, Ehrlich R, Sherf I et al. Diode laser treatment of retinopathy of prematurity: anatomical and refractive outcomes. Retina 2008; 28: 839–846.
Dhawan A, Dogra M, Vinekar A, Gupta A, Dutta S . Structural sequelae and refractive outcome after successful laser treatment for threshold retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 2008; 45: 356–361.
Quinn GE, Dobson V, Kivlin J, Kaufman LM, Repka MX, Reynolds JD et al. Prevalence of myopia between 3 months and 5 1/2 years in preterm infants with and without retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1998; 105: 1292–1300.
Sahni J, Subhedar NV, Clark D . Treated threshold stage 3 versus spontaneously regressed subthreshold stage 3 retinopathy of prematurity: a study of motility, refractive, and anatomical outcomes at 6 months and 36 months. Br J Ophthalmol 2005; 89: 154–159.
Algawi K, Goggin M, O'Keefe M . Refractive outcome following diode laser versus cryotherapy for eyes with retinopathy of prematurity. Br J Ophthalmol 1994; 78: 612–614.
Mintz-Hittner HA, Kennedy KA, Chuang AZ . Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011; 364: 603–615.
Harder BC, von BS, Schlichtenbrede FC, Jonas JB . Early refractive outcome after intravitreous bevacizumab for retinopathy of prematurity. Arch Ophthalmol 2012; 130: 800–801.
Harder BC, Schlichtenbrede FC, von BS, Jendritza W, Jendritza B, Jonas JB . Intravitreal bevacizumab for retinopathy of prematurity: refractive error results. Am J Ophthalmol 2013; 155: 1119–1124.
Castellanos MA, Schwartz S, Garcia-Aguirre G, Quiroz-Mercado H . Short-term outcome after intravitreal ranibizumab injections for the treatment of retinopathy of prematurity. Br J Ophthalmol 2013; 97: 816–819.
Tseng CC, Chen SN, Hwang JF, Lin CJ . Different refractive errors in triplets with retinopathy of prematurity treated with bevacizumab. J Pediatr Ophthalmol Strabismus 2012; 49: e41–e43.
Section on Ophthalmology American Academy of Pediatrics; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2006; 117: 572–576.
An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol 1984; 102: 1130–1134.
Good WV . Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004; 102: 233–248.
Repka MX . Refraction and keratometry in premature infants. Br J Ophthalmol 2004; 88: 853–854.
Martinez-Castellanos MA, Schwartz S, Hernandez-Rojas ML, Kon-Jara VA, García-Aguirre G, Guerrero-Naranjo JL et al. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina 2013; 33: 329–338.
Chen TC, Tsai TH, Shih YF, Yeh PT, Yang CH, Hu FC et al. Long-term evaluation of refractive status and optical components in eyes of children born prematurely. Invest Ophthalmol Vis Sci 2010; 51: 6140–6148.
Wu WC, Lin RI, Shih CP, Wang NK, Chen YP, Chao AN et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology 2012; 119: 1907–1916.
van Heuven WA, Kiel JW . ROP surgery and ocular circulation. Eye (Lond) 2008; 22: 1267–1272.
Holz ER . Refractive outcomes of three-port lens-sparing vitrectomy for retinopathy of prematurity (An AOS Thesis). Trans Am Ophthalmol Soc 2009; 107: 300–310.
Holmström M, el Azazi M, Kugelberg U . Ophthalmological long-term follow up of preterm infants: a population based, prospective study of the refraction and its development. Br J Ophthalmol 1998; 82: 1265–1271.
Acknowledgements
The authors have no proprietary interest in any aspect of this study and received no government funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, YH., Chen, SN., Lien, RI. et al. Refractive errors after the use of bevacizumab for the treatment of retinopathy of prematurity: 2-year outcomes. Eye 28, 1080–1087 (2014). https://doi.org/10.1038/eye.2014.172
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.172
This article is cited by
-
Outcomes of near confluent laser versus combined less dense laser and bevacizumab treatment of prethreshold ROP Type 1 Zone 2: a randomized controlled trial
BMC Ophthalmology (2022)
-
Refractive status, biometric components, and functional outcomes of patients with threshold retinopathy of prematurity: systemic review and a 17-year longitudinal study
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Predictors and ocular outcomes of rescue treatment in preterm infants with treated retinopathy of prematurity—a retrospective study
Eye (2021)
-
Retinopathy of prematurity treatment: Asian perspectives
Eye (2020)
-
Neurodevelopmental outcomes in infants treated with intravitreal bevacizumab versus laser
Journal of Perinatology (2019)