Abstract
Purpose
Central serous chorioretinopathy (CSCR) is an idiopathic disorder characterised by detachment of the neurosensory retina due to serous fluid accumulation between the photoreceptor outer segments and the retinal pigment epithelium. There are currently no set guidelines or protocols on its treatment. This study was undertaken to assess the current literature on the the efficacy and safety of photodynamic therapy (PDT) as a treatment option for CSCR.
Methods
Seven databases (PubMed, CENTRAL, MEDLINE, Web of Science, Embase, Scopus, and The Cochrane Database of Systematic Reviews) were searched without restrictions on time or location. We followed PRISMA guidelines and evaluated quality according to STROBE criteria. In total, 117 citations were identified and 31 studies describing 787 eyes were included for review. Data on indications for PDT in CSCR, dosing regimens of verteprofin PDT (which includes treatment dose of vertoporfin, treatment time, fluence, and spot size), number of treatment sessions, response to treatment, mean length of follow-up, and complications were extracted and analysed.
Results
Since the introduction of PDT for the treatment of CSCR in 2003, there have been three randomised controlled trials (RCTs), one for acute and two chronic CSCR and 28 further studies that met the STROBE criteria that compared the use of PDT with other treatment options. All studies showed short-term efficacy of PDT in CSCR. The studies were of small sample size and lacked sufficient follow-up to draw conclusions on long-term efficacy and safety.
Conclusions
There is sufficient scientific evidence to suggest that PDT may be a useful treatment option for chronic CSCR in the short-term. The review identifies a need for robust RCTs with longer follow-up to ascertain the role of PDT as a useful treatment option for CSCR.
Similar content being viewed by others
Introduction
While the exact pathophysiologic mechanisms of central serous chorioretinopathy (CSCR) remains unknown, CSCR is thought to be a primary disorder of choroidal permeability from possible inflammation, ischaemia, or stasis.1, 2, 3 The advent of fluorescein angiography (FA) and indocyanine green angiography (ICG-A) has helped in the diagnosis of this often debilitating condition. Typical FA findings include one or two areas of focal juxtafoveal focal leakage at the level of the RPE, with ‘inkblot’ or ‘smokestack’ hyperfluorescence. ICG-A, which facilitates evaluation of the choroidal vasculature, highlights mid-phase multifocal areas of choroidal hyperfluorescence in CSCR patients.4, 5 These areas are postulated to be caused by choroidal vascular hyperpermeability.4, 6, 7, 8
Over the last two decades, numerous treatments for chronic CSCR have been investigated. These have included pharmacologic therapy, laser photocoagulation, photodynamic therapy (PDT) and most recently anti-vascular endothelial growth factor (anti-VEGF).4, 5, 7, 8, 9, 10, 11, 12, 13 Each study with different treatment modalities has had its own drawbacks—small population size, treatment efficacy, and adverse event occurrence. In this review, we assess the studies that evaluated the use of PDT in the treatment of CSCR.5, 7, 8
In 1999, the treatment of age-related macular degeneration with photodynamic therapy (TAP) study group conducted a randomised control trial which showed superiority with the use of verteporfin vs placebo to treat wet age-related macular degeneration (P<0.01). The standard regime consisted of a light sensitive drug, verteporfin, (6 mg/m2) which is administered via intravenous infusion of 30 ml over 10 min. Fifteen minutes after the start of the infusion, a laser light at 689 nm delivered 50 J/cm2 at an intensity of 600 mW/cm2 over 83 s using a spot size with a diameter 1000 μm larger than the greatest linear dimension of the choroidal neovascularization (CNV) lesion.9 Over the past decade, there have been various modifications of the PDT regime in terms of fluence, verteporfin dose and time that have been tried. To our knowledge, there has been only one systematic review by Karim and Adelman,5 who assessed the use of various verteporfin doses in PDT as a treatment option for CSCR. Other variations of PDT treatment have not undergone a systematic review. The rationale of using various PDT modifications has been to reduce both the side effects of PDT and reduce the number of recurrences of CSCR. The main objective of this study was to evaluate whether current literature can guide us regarding PDT's efficaciousness in treating CSCR and what are the best PDT parameters to use.
Materials and methods
We conducted a systematic search of seven databases: PubMed, CENTRAL, MEDLINE, Web of Science, Embase, Scopus, and The Cochrane Database of Systematic Reviews (from 2003 to present) for randomised controlled trials (RCTs) and all other clinical studies with at least 1-month follow-up. All prospective and retrospective studies that met the STROBE criteria were included. The search strategy used both keywords and MeSH terms for the following terms or combinations: Central serous retinopathy, CSCR, PDT, verteporfin; anti-VEGF; ranibuzimab; bevacizumab.
All RCTs were included in our analysis. We included studies that met at least 70% of the STROBE standards (See table 1 below). Two reviewers assessed inclusion into this study and consensus was reached by discussion between reviewers.
The primary parameters of interest were as follows:
-
1
Indications for PDT based on various definitions of CSCR.
-
2
Dose of verteporfin, fluence, spot size, treatment time, and frequency of treatment.
-
3
Mean change in best corrected visual acuity (BCVA).
-
4
Change in macular thickness on optical coherence tomography (OCT).
-
5
Changes in choroidal thickness.
-
6
Changes of leakage on fundus fluorescein angiography (FFA).
-
7
Length of follow-up.
-
8
Complications such as choroidal non-perfusion and choroidal neovascularisation.
In the case of studies that sequentially reported longer follow-up of the same series of patients, the latest study with the longest follow-up was included. Data were entered into a Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) datasheet for tabulation and descriptive statistics.
Results
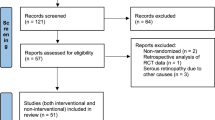
A thorough electronic database search yielded a total of 117 publications that reported the clinical outcomes of PDT in the treatment of CSCR.
PDT and CSCR
A total of 31 reports on clinical outcomes met the inclusion criteria for the use of PDT in CSCR/CSC treatment (see Figure 1).11, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 These included three RCTs (Bae et al, Semeraro et al and Chan et al) that compared PDT with other treatment options as well as 28 STROBE-qualified studies, which also evaluated the efficacy of using PDT to treat CSCR.11, 15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 41, 42, 43, 44 A summary of the three RCTs have been tabulated in Table 2. A meta-analysis could not be performed because of the heterogeneity of the studies.
STROBE-qualified studies
Twenty-eight studies met at least 70% of the STROBE criteria.11, 15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 41, 42, 43, 44 The total number of eyes in the studies varied from 5 to 82 (mean 25). Eleven of these studies used standard TAP protocol. The remaining seventeen studies assessed the effect of different parameter variations in the standard TAP protocol in treating CSCR: reduced fluence (eight studies), reduced dose verteporfin (eight studies), variation in exposure time (one study). Two studies17, 22 were noted to have more than one variation in standard TAP protocol both of which used half-dose verteporfin and decreased irradiation time.
Acute vs Chronic CSCR
Acute CSCR was diagnosed if symptoms lasted <3 months, whereas chronic CSCR was diagnosed if symptoms persisted over 3 months.11, 15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 41, 42, 43, 44 Three studies contained the use of PDT in treating acute CSCR (n=3), whereas the remaining 28 studies assessed the efficacy of using PDT to treat chronic CSCR (n=28).11, 15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 41, 42, 43, 44
Acute CSCR.
Of our three studies identified, one was an RCT and the other two were case series. Two studies used reduced dose of verteporfin (decreasing dose by 10% from 6 mg and half-dose verteporfin (3 mg)) compared with the standard dose (6 mg), whereas the other used half-fluence PDT (25 J/cm2 compared with 50 J/cm2).16, 32, 39
RCT: Chan et al16 in 2008 compared the use of reduced dose verteporfin (3 mg) vs Placebo in 63 eyes. The other standard TAP parameters remained unchanged. The mean duration of CSCR was 6.4±1.7 weeks. All patients had only one treatment session and the mean±SD PDT laser spot size was 4200±565 μm (range, 3400–4500 μm). Fifty-eight of the 63 patients (92.1%) completed 12-month follow-up.16 (See Table 2 below.)
Outcome measures
Mean BCVA.
Three months post treatment, the mean ±SD logMAR BCVA of the verteporfin group improved to 0.00±0.11 (Snellen equivalent, 20/20), whereas the placebo group improved to 0.08±0.11 (Snellen equivalent, 20/24) from 0.16±0.19 (20/29) and 0.11±0.12 (20/26), respectively (P=0.015). The mean logMAR BCVA at 12 months was significantly better in the verteporfin group compared with the placebo group: – 0.05±0.09 (Snellen equivalent, 20/18), vs placebo group of 0.05±0.17 (Snellen equivalent, 20/22) (P=0.008). The mean lines of BCVA improvement at 1 year for the verteporfin group was 1.8 lines, compared with 0.6 line for the placebo group (P=0.002).16
Resolution of subretinal fluid (SRF).
At the 3-month visit, 35 (89.7%) eyes in the verteporfin group had absence of subretinal fluid, compared with 8 (42.1%) eyes in the placebo group (P=0.001). At 6 months, 36 (92.3%) eyes in the verteporfin group had absence of subretinal fluid, compared with 11 (57.9%) eyes in the placebo group (P=0.003). Thirty-seven (94.9%) eyes in the verteporfin group compared with 11 (57.9%) eyes in the placebo group showed absence of subretinal fluid at the macula at 12 months (P=0.001).16
Central foveal thickness (CFT) on OCT.
The baseline mean±SD OCT CFT for the verteporfin and placebo groups were 456±223 μm and 452±218 μm respectively. At 3 months, the mean±SD OCT CFT of the verteporfin group reduced to 165±82 μm, compared with 309±182 μm for the placebo group (P<0.001), while at 12 months, the mean±SD OCT CFT for the verteporfin group remained significantly lower compared with the placebo group, with 161±65 μm and 278±192 μm, respectively (P=0.001).16
Fluorescein leakage post PDT using ICG-A.
At 3 months post PDT treatment, 34 (87.2%) of 39 eyes in the verteporfin group showed complete absence of fluorescein leakage compared with only 4 (12.8%) of 19 eyes in the placebo group (P<0.001).16
STROBE Studies: Zhao et al39 assessed the use of decreasing dose of verteporfin in treating acute CSCR.15 eyes were assessed. The mean duration before treatment was 35 days (range, 5 days to 4.5 months). The mean follow-up was 11.8 months (range, 6–16 months). The mean number of treatment sessions was 1.25. The mean PDT laser spot size was 2400 μm (range, 500–3700 μm).
Smertsching et al32 assessed the use of half-fluence PDT in treating acute CSCR. 19 eyes were assessed in the study. The duration of symptoms was 31.7± 38.1 days. Fifteen eyes were followed up to 12 months (79%). Only one treatment session was required.
Outcome measures
Mean BCVA.
Both studies reported an improvement in the mean BCVA after treatment for acute CSCR, which were statistically significant. In Zhao et al39 the mean±SD logMAR BCVA improved from 0.47±0.28 before PDT to 0.05±0.19. A 4.1±0.25 line improvement 12 months after follow-up was reported. Similarly, Smertsching et al32 also reported an improvement in vision. Sixteen eyes were evaluated at month 3, and the BCVA score changed from baseline by a mean of 10 letters (P<0.002). Six months after PDT, 16 eyes were evaluated, and the BCVA score showed an improvement compared with baseline by a mean of 10 letters (P<0.001). At 12-month follow-up, 15 eyes were evaluated and BCVA score has changed by nine letters (from 47 to 56) on the ETDRS chart at 12 months (P=0.003).
CFT on OCT.
One month post PDT treatment, Smertsching et al32 reported complete resolution of SRF on spectral-domain OCT in all 19 patients. By the 12th month follow-up, CFT had decreased by a mean of 163 μm from 406 μm (P<0.001).
Chronic CSCR.
Two RCTs and 26 STROBE-qualified studies were identified from our search.11, 15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 41, 42, 43, 44 Eleven of these studies used normal TAP protocol (n=11). The remaining fifteen studies assessed the effect of different parameter variations in the standard TAP protocol in treating CSCR: reduced fluence (seven studies), reduced dose verteporfin (seven studies), variation in exposure time (one study). Two studies (Chan et al and Nicolo et al) were noted to have more than one variation in standard TAP protocol both of which used half-dose verteporfin and decreased irradiation time.
RCT: Bae et al40 undertook a randomized study on the efficacy of half-fluence PDT vs intravitreal ranibizumab (0.5 mg/0.05 ml monthly for three months) to treat chronic CSCR in 16 eyes (eight eyes to each group). The mean duration of CSCR was 28.9±23.6 months. The mean number of treatments was 1.25 sessions in the half-fluence group (a total of 1.38 sessions in both groups). Rescue treatments with a single session of low-fluence PDT for the ranibizumab group and ranibizumab injection for the low-fluence PDT group were conducted if there was re-accumulation or sustained SRF during the subsequent follow-up period and BCVA was <0.2 LogMar. The mean PDT laser spot size was 1778.94±464.97 μm; range, 1500–3100 μm). All 16 eyes completed 6-months follow-up.
Semeraro et al undertook a randomized study on the efficacy of half-fluence PDT vs intravitreal bevacizumab (1.25 mg once off) to treat chronic CSCR in 22 eyes (12 eyes in bevacizumab group and 10 eyes in low-fluence PDT group). The mean duration of CSCR was >3 months. The mean number of treatments was 1.6±0.6 sessions in the half-fluence group and 3±1 injections in the anti-VEGF group. Fifty percent of all eyes seen had a recurrence of CSCR post initial treatment (seven eyes in anti-VEGF group and four eyes in low-fluence PDT group). Re-injections of bevacizumab or re-treatment with low-fluence PDT were scheduled at least 4 weeks after the initial treatment if one or both of the following criteria were met:
-
1
Decrease in BCVA of at least five letters on two repeated tests associated with an increase in the pooling area on FA; and/or
-
2
No decrease in intra-retinal fluid or pigment epithelial detachment (PED) documented by OCT.14
The mean PDT laser spot size was not documented and all 22 eyes completed 9-months follow-up. No complications were documented.
Outcome measures
Mean BCVA.
Bae et al40 showed that patients treated with low-fluence PDT had an improvement in the mean BCVA: improved from 0.30±0.37 at baseline to 0.18±0.27 at 3 months, but this was not statistically significant (P=0.075) compared with their ranibizumab group. At the 6-month follow-up, there was further improvement in BCVA to 0.13±0.17 in the PDT arm, yet this was still not statistically significant.
In Semeraro et al’s study, the mean visual acuity (VA) score (number of ETDRS letters read) improved from 20 ETDRS letters (SD11) to 43 letters (SD 14) at 9 months (P=0.032) in the anti-VEGF group. In the low-fluence PDT group, the mean VA score improved from 30 ETDRS letters (SD 8) to 40 letters (SD 12) at 9 months (P=0.028). Although the improvement in VA was greater in the anti-VEGF group compared with the low-fluence PDT group, the result was not statistically significant. (P=0.59).14
SRF.
In 75% of the half-fluence group (40), there was complete resolution of SRF at 6 months compared with only 25% of eyes in the ranibizumab group.
CFT on OCT.
In Bae et al’s40 low-fluence PDT group, the mean excess foveal thickness was reduced significantly from 74.1±56.0 μm at baseline to −35.4±44.5 μm at 3 months (P=0.017) compared with the ranibizumab group (mean excess foveal thickness decreased from 26.3±50.6 μm at baseline to −23.1±56.5 μm at 3 months).
In Semeraro et al’s study, OCT revealed a decrease in the serous detachment and the focal areas of PED in both groups. The mean change over 9 months from baseline in central point thickness, defined as the distance between the Bruch membrane and the inner retinal surface, was 127 μm (SD 36) in the anti-VEGF group and 114 μm (SD 42) in the low-fluence PDT group (P=0.0027 and P=0.0031, respectively). Overall, the macular thickness in all patients significantly decreased during the follow-up period (from 345±85 μm in the anti-VEGF group to 218±42 and from 361±108 μm in the low PDT group to 247±32 μm, respectively).14
Leakage on FFA.
In Bae et al’s40 study, 12 eyes were noted to have active leakage on FFA prior to treatment commencement in both groups: 6 in the low-fluence PDT group and 6 in the ranibizumab group. After completion of primary treatment, five eyes in the ranibizumab group showed persistent active leakage on FFA with only moderate reduction. At 6 months, persistent leakage was regressed in four eyes after an additional low-fluence PDT. All eyes in the low-fluence PDT group demonstrated complete resolution of active leakage after primary treatment, regardless of the presence of SRF.
In Semeraro et al’s study, the area of FA pooling decreased in both groups. The difference between FA area size at baseline and that at the nine-month-follow-up visit was statistically significant (anti-VEGF group: P=0.001; low-fluence PDT group: P=0.01), although the difference between the two groups was not significant. (P=0.56).14
STROBE studies: Pooled data from STROBE-selected studies showed the number of eyes in the studies varied between 5 and 82 eyes (mean: 24 eyes). The mean duration of chronic CSCR varied from 3 months to 16 years and the mean follow-up duration in these studies ranged from 1 to 56.8 months. Five studies (20.8%) had <6-month follow-up.11, 15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 33, 34, 35, 36, 37, 38, 39, 41, 46, 47 Table 3 below highlights key findings from the papers.
Variation in treatment parameters
Standard PDT.
Eleven studies (n=11) used standard TAP protocol as discussed previously above to treat chronic CSCR. In all ten studies, there was a statistically significant improvement in BCVA after treatment (P<0.05).23, 25, 26, 30, 33, 34, 36, 37, 41, 43, 44, 46
Fluence.
Of the seven studies identified, three fluence-dosing regimens were identified: Standard fluence (50 J/cm2), half fluence (25 J/cm2) and quarter fluence (12 J/cm2). Two studies (n=2) compared standard fluence (50 J/cm2) with half-fleunce (25 J/cm2), whereas four studies (n=4) used half fluence (25 J/cm2) and one study (n=1) used a quarter fluence (12 J/cm2) alone to treat CSCR.15, 18, 20, 24, 27, 31
Shin et al27 and Reibaldi et al compared standard vs half-fluence PDT. Both authors reported better BCVA in the half-fluence group at the end of 12-month follow-up compared the standard group. However, Reibaldi et al24 noted a further reduction CFT in the half-fluence group compared with the Standard fluence group, whereas Shin et al27 noted the opposite. Similarly in the other four studies, BCVA improved and was statistically significant at the end of follow-up.
Verteporfin dose.
The doses of verteporfin administered varied from 2 mg/m2 to 6 mg/m2, although most studies used a reduced dose of 3 mg/m2 (n: 7).17, 19, 21, 22, 28, 35, 42, 47 All studies noted an improvement in the BCVA after treatment with verteporfin at the end of follow-up, as well as a reduction in CFT/ central macular thickness (CMT) on OCT. Uetani et al35 in 2012 compared the use of 2 mg/m2 vs 3 mg/m2 verteporfin to treat CSCR in 16 eyes. At the end of the 3-month follow-up period, the BCVA was found to be better in the 3 mg/m2 group compared with the 2 mg/m2 group, although this was not statistically significant. From the studies analysed, we can deduce that 3 mg of verteporfin (half dose) produced the best results in terms of BCVA and reduction in CMT after a 6-month follow-up period.
Time.
Only one study (n=1) had a variation in time: Pyrds et al42 with a light exposure time of only 42 s (as against 83 s). Complete resorption of the subretinal fluid was documented in all eyes at the 1-month follow-up. BCVA improved from 0.13 LogMar to 0.06 LogMar following a treatment after a 1-month follow-up period. Fourteen patients reported subjective visual improvement in the form of reduction or elimination of the relative central scotoma and or metamorphopsia. Choroidal thickness in the area where PDT was applied decreased from 407 μm (mean; 95% confidence interval (CI) 356–458 μm) to 349 μm (mean; CI95 300–399 μm; P<0.0001), and subfoveal choroidal thickness was reduced from 421 μm (mean; CI95 352–489 μm) to 346 μm (mean; CI95 278–414 μm; P=0.0001). Initially, subfoveal choroidal thickness was significantly increased in the treated eye compared with the healthy fellow eye (mean 324 μm; CI95 273–376 μm; P=0.0003), but after treatment, the difference was not significant.
Combination of treatment parameters.
Chan et al17 and Nicolo et al22 had more than one variation in standard TAP protocol: both used half-dose verteporfin and decreased irradiation time. Both studies reported a significant improvement in BCVA and mean decrease in CFT after treatment with PDT.
Laser spot size.
Mean laser spot size ranged between 1300 and 6800μm.15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30, 31, 33, 34, 35, 36, 37, 39, 41, 42, 43, 44, 46, 47 Thirteen studies (n=13, 54.2%) used a mean spot size <4500 μm. The spot size was not mentioned in seven studies.
Treatment sessions.
The mean number of treatment sessions varied from 1 to 1.8 sessions. Fifteen studies (n=15, 62.5%) had only one PDT treatment session and five (n=5, 20.8%) had 1.1 sessions. The mean number of sessions was 1.1. Recurrence of SRF was the main indicator for the second treatment. The time to recurrence ranged from 3 to 22 months in all studies analysed, with most recurrences happening 3–6 months after initial PDT treatment. Other important factors contributing to re-treatment were persistent SRF and development of CNV following the first treatment, as seen in Reibaldi et al’s study. Time to second treatment in eyes undergoing re-treatment was on average 6 months (range from 3 to 12 months).15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30, 31, 33, 34, 35, 36, 37, 39, 41, 42, 46, 47
Outcome measures
BCVA.
Although studies are heterogeneous, from Table 1 we can deduce an improvement in BCVA at 1,3, 6, and 12 months in all groups. At 21.9 months, Tarantola et al34 found an improvement in LogMar BCVA from 0.34 to 0.24.
SRF.
At 1 month post PDT treatment, the percentage of SRF resolution varied from 70 to 100%. Only three studies reported complete SRF resolution at 1 month. At 12 months, SRF resolution had improved to 75–100% in all studies. All the other studies had >90% SRF resolution at 12 months.15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30, 31, 33, 34, 35, 36, 37, 39, 41, 42, 43, 44, 46, 47 Below is an analysis of the variations in PDT treatment parameters and the effects on SRF resolution.
Standard PDT group: Of the 11 studies analysed, 4 (36%) had complete resolution of SRF and no recurrence of SRF during the follow-up period. In Chan et al’s study, one juxtafoveal CNV developed 3 months post standard PDT treatment.23, 25, 26, 30, 33, 34, 36, 37, 41, 46
Variation in verterporfin dose: The variation in verteporin dose PDT group had the best results in terms of resolution of SRF and improvement of BCVA post PDT treatment. In the half-dose verterporfin group, complete resolution of SRF was seen in three out of seven studies (42.9%) at the end of each study’s follow-up period. Uetani et al reported 70% resolution of SRF in the half-dose verterporfin group compared with only 33% in the 2 mg/m2 verterporfin group 1 month after treatment with PDT. 100% resolution of SRF was seen in both half-dose and one-third-dose verterforin though, 3 months after treatment. In Koytak et al’s study, complete resolution of SRF was noticed in six of eight eyes (75%) at the end of 12 months. No complications were noted in the decreased-dose verterporfin group following PDT treatment.17, 19, 21, 22, 29, 35, 47
Variation in Fluence: It is interesting to note that all of the studies with a variation in fluence had a recurrence of CSCR post PDT treatment within a 1-year-follow-up period. Recurrence rate of CSCR varied from 3 to 24%.15, 18, 20, 24, 27, 31 Shin et al’s27 reported the best results in terms of resolution of CSCR with a 91.1% resolution of SRF in the half-fluence group compared with 97.0% in the standard fluence group at 1 month. There was only one (3%) recurrence of CSCR in the half-fluence group during the 10-year follow-up period. Inuoe et al18 had the worst CSCR recurrence rates. SRF had completely resolved in 29 eyes (91%) at 3 months after one application of PDT but recurrence of CSCR 12 months post CSCR was seen in 7 of 29 eyes (24%). In Reibaldi et al’s24 study, a juxtafoveal CNV developed 3 months after treatment in one standard-fluence-treated eye.
OCT and Anatomic Changes after PDT using CFT or CMT measurements
Table 4 below shows the results of resolution of SRF in the STROBE-qualified studies. From our research, 19 studies assessed the resolution of SRF using CFT (n=16) or CMT (n=3) as a marker. In all studies although not heterogeneous, there was a statistically significant reduction in either CMT or CFT after treatment with PDT at 1, 3, 6, and 12 months. Tarantola et al also noticed SRF resolution and a reduction in CFT at 24 months (231±94 μm from 375±62 μm). A similar pattern was noticed in Silva et al at 48 months with a reduction in CMT from 316±114 to 169.7±41.1 μm.15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30, 31, 33, 34, 35, 36, 37, 46, 47
Choroidal thickness on OCT
Three studies assessed the choroidal thickness before and after PDT treatment (See Table 5 below).22, 41, 42 Both Maruko et al21 and Pyrds and Larsen42 demonstrated that choroidal thickness in the area treated with PDT initially increased after treatment, then decreased. Pyrds and Larsen showed that choroidal thickness in the area where PDT was applied decreased from 407 μm (mean; 95% CI 356–458 μm) to 349 μm (mean; CI 95% 300–399 μm; P<0.0001), and subfoveal choroidal thickness was reduced from 421 μm (mean; CI 95% 352–489 μm) to 346 μm (mean; CI 95% 278–414 μm; P=0.0001). They also demonstrated that initially, subfoveal choroidal thickness was significantly increased in the treated eye compared with the healthy fellow eye (mean 324 μm; CI 95% 273–376 μm; P=0.0003), but after treatment, the difference was not significant. A similar result is seen in Maruko et al’s21 study where the mean choroidal thickness in the PDT group increased significantly from 389±106 μm at baseline to 462±124 μm (P=0.008) by 2 days after treatment, and then reduced rapidly to 360±100 μm (P=0.001) at 1 week and 330±103 μm (P=0.001) after 4 weeks as compared with baseline. In Chan et al41 3 months after PDT, where the mean diameter of the dilated choroidal vessel reduced from 546 μm to 371 μm (P=0.028) in the six eyes analysed.
Recurrence of CSCR
From Table 2, ten studies (n=10) recorded no recurrence of SRF after treatment with PDT in a 12-month follow-up period. We can also deduce that the recurrence rate of CSCR once treated with PDT varied from 0 to 24% despite previous complete resolution of SRF after first treatment (n=14). Recurrence of CSCR resulted in another session of PDT treatment in most studies.15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30, 31, 33, 34, 35, 36, 37, 39, 41, 42, 46, 47 There was no pattern to timing of recurrence in any of the studies.
Complications
Eighty-five percent of studies (n=22) reported no ocular or systemic complications from the administration of PDT in the treatment of CSCR.15, 16, 17, 18, 19, 20, 21, 22, 23, 26, 27, 28, 30, 31, 33, 34, 35, 36, 37, 43, 44, 46, 47 Four studies, however, reported ocular complications.24, 25, 41, 44 Ruiz–Moreno et al25 reported secondary CNV in two eyes (2.4%) post PDT treatment. Arevalo et al as well as both Chan et al41 and Reibaldi et al24 also noted the development of juxtafoveal CNV in one eye each (5.6%, 16.7%, and 2.4% respectively) three months after PDT treatment.
Discussion
Chronic and recurrent CSCR can be a debilitating condition, often affecting the working age group. There is currently no gold standard therapy for its treatment. In this systematic review, we evaluated the efficacy of using standard and varied PDT treatment modalities from various RCTs and STROBE-qualified observational studies over the last 10 years. All these studies had small sample sizes, different inclusion criteria, different methods of examination, short follow-up and lacked matched controls. All treatment modalities led to an improved BCVA and a resolution of SRF to varying degrees of success; however, no correlation could be established between BCVA and CFT before or after PDT in any study. This lack of correlation may be because of the fact that majority of our studies analysed had a mean duration of CSCR>6 months prior to PDT therapy, by which time photoreceptors may have been damaged.
There are two RCTs that compared PDT with other treatment modalities in treating CSCR in both the acute and chronic forms at six months. Although the verteporfin and fluence doses varied between these two studies, the mean BCVA at 3 months was better in both groups (P=0.015 and P=0.075 respectively). This effect was maintained at 6 months in both studies. A similar effect was also noted in the STROBE-qualified studies.
Our review suggest a lower rate of side effects and reduction in CSCR recurrence for eyes treated with half-dose verteporfin PDT. In the half-dose verterporfin group, complete resolution of SRF without recurrence was seen in three out of seven studies (42.9%) at the end of each study’s follow-up period. A converse effect was seen in the variable-fluence group, where all of the studies with a variation in fluence had a recurrence of CSCR post PDT treatment within a 1-year-follow-up period. Recurrence rate of CSCR varied from 3 to 24%.
In most of our analysed studies, the laser treatment spot size was selected based on angiographic leakage in the FA and not on choroidal abnormality as demonstrated on ICG-A, hence, why a smaller laser spot was used in treating majority of cases. This approach was to avoid overtreatment of the choroidal vasculature and prevent choroidal ischaemia. However, it may be argued that the pathological level of CSCR is actually at the choroidal level, so treatment may be better guided by ICG-A findings and not by leakages sites on FA.
PDT treatment is known to be associated with certain rare but serious complications: such as secondary RPE changes, choriocapillaris hypoperfusion, choroidal ischaemia, choroidal infarction, and CNV development related to the hypoxic damage caused by choriocapillaris occlusion at the site of PDT, all of which can potentially reduce the VA.11, 16, 46, 48 As CNV is a known complication of CSCR, it is difficult to assess the role of PDT in the development of CNV. In three of our analysed studies, two patients developed juxtafoveal CNV 3 months after PDT treatment. A further two patients developed secondary CNV during follow-up. In these cases, a decrease in choroidal perfusion could have increased the risk of CNV development by promoting release of vascular endothelial growth factor. There were no documented cases of choroidal non-perfusion.
Conclusion
Our systematic review demonstrates that a modified half-dose PDT protocol remains the safest and most effective method of treating chronic CSCR based on a heterogeneous collection of studies. Ideally, a randomised controlled clinical trial is warranted to evaluate the efficacy of modified PDT regime using half-dose verteporfin in treating patients with chronic CSRC with other available treatment options to better understand the relative effects of the various options available for this condition.
References
Gass JDM . Pathogenesis of disciform detachment of the neuroepithelium. II. Idiopathic central serous choroidopathy. Am J Ophthalmol. 1967; 63: 587–615.
Maumenee AE . Symposium: Macular diseases. Clinical manifestations. Trans Am Acad Ophthalmol Otolaryngol 1965; 69: 605–613.
von Graefe A . Kurzere Abhandlungen, Notizen and casuistische Mittheilungen vermischten Inhalts. VI. Ueber centrale recidivirende Retinitis Albrecht von Graefes. Arch Ophthalmol. 1866; 12 (Pt. II): 2115.
Ferrara Daniela C, Calucci Daniela, Oréfice Juliana, Magalhães Érika P, Oréfice Fernando, Costa Rogerio A . Proposed physiopathological mechanisms and potential therapeutic targets for central serous chorioretinopathy. Expert Rev Ophthalmol 2008; 3 (5): 553–565.
Karim S, Adelman R . Profile of verteporfin and its potential for the treatment of central serous chorioretinopathy. Clin Ophthalmol 2013; 2013 (default): 1867.
Eandi CM, Ober M, Iranmanesh R, Peiretti E, Yannuzzi LA . Acute central serous chorioretinopathy and fundus autofluorescence. Retina 2005; 25 (8): 989.
Gupta B, Mohamed MD . Photodynamic therapy for variant central serous chorioretinopathy: efficacy and side effects. Ophthalmologica 2011; 225 (4): 207–210.
Nicholson B, Noble J, Forooghian F, Meyerle C . Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol 2013; 58 (2): 103–126.
Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch ophthalmol 1999; 117 (10): 1329.
Chan W-M, Lam DSC, Lai TYY, Yuen KSC, Liu DTL, Chan CKM et al. Treatment of choroidal neovascularization in central serous chorioretinopathy by photodynamic therapy with verteporfin. Am J Ophthalmol 2003; 136 (5): 836–845.
Lai TYY, Chan WM, Li H, Lai RYK, Liu DTL, Lam DSC . Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol 2006; 90 (7): 869.
Mennel S, Barbazetto I, Meyer CH, Peter S, Stur M . Ocular photodynamic therapy – standard applications and new indications (Part 1). Ophthalmologica 2007; 221 (4): 216–226.
Tsai D-C, Chen S-J, Huang C-C, Chou P, Chung C-M, Huang P-H et al. Epidemiology of idiopathic central serous chorioretinopathy in Taiwan, 2001-2006: a population-based study. PloS One 2013; 8 (6): e66858.
Semeraro F, Romano MR, Danzi P, Morescalchi F, Costagliola C . Intravitreal bevacizumab versus low-fluence photodynamic therapy for treatment of chronic central serous chorioretinopathy. Jpn J Ophthalmol 2012; 56 (6): 608–612.
Butler AL, Fung AT, Merkur AB, Albiani DA, Forooghian F . Very minimal fluence photodynamic therapy for chronic central serous chorioretinopathy. Can J Ophthalmol 2012; 47 (1): 42–44.
Chan W-M, Lai TYY, Lai RYK, Liu DTL, Lam DSC . Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology 2008; 115 (10): 1756–1765.
Chan W-M, Lai TYY, Lai RYK, Tang EWH, Liu DTL, Lam DSC . Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina 2008; 28 (1): 85.
Inoue R, Sawa M, Tsujikawa M, Gomi F . Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am J Ophthalmol 2010; 149 (3): 441–446 e2.
Koytak A, Erol K, Coskun E, Asik N, Öztürk H, Özertürk Y . Fluorescein angiography-guided photodynamic therapy with half-dose verteporfin for chronic central serous chorioretinopathy. Retina 2010; 30 (10): 1698.
Lim SH, Chang W, Sagong M . Efficacy of half-fluence photodynamic therapy depending on the degree of choroidal hyperpermeability in chronic central serous chorioretinopathy. Eye 2013; 27 (3): 353.
Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF . Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology 2010; 117 (9): 1792–1799.
Nicolò M, Zoli D, Musolino M, Traverso CE . Association between the efficacy of half-dose photodynamic therapy with indocyanine green angiography and optical coherence tomography findings in the treatment of central serous chorioretinopathy. Am J Ophthalmol 2012; 153 (3): 474–480 e1.
Özmert E, Batioglu F . Fundus autofluorescence before and after photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmologica 2009; 223 (4): 263–268.
Reibaldi M, Cardascia N, Longo A, Furino C, Avitabile T, Faro S et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol 2010; 149 (2): 307–315 e2.
Ruiz-moreno JM, Lugo FL, Armadá F, Silva R, Montero JA, Arevalo JF et al. Photodynamic therapy for chronic central serous chorioretinopathy. Acta Ophthalmologica 2010; 88 (3): 371–376.
Sakalar YB, Keklikci U, Unlu K, Alakus MF, Kara IH . Effects of photodynamic therapy with verteporfin for the treatment of chronic central serous chorioretinopathy: an uncontrolled, open-label, observational study. Curr Ther Res Clin Exp 2010; 71 (3): 173–185.
Shin JY, Woo SJ, Yu HG, Park KH . Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina 2011; 31 (1): 119.
Shinojima A, Hirose T, Mori R, Kawamura A, Yuzawa M . Morphologic findings in acute central serous chorioretinopathy using spectral domain-optical coherence tomography with simultaneous angiography. Retina 2010; 30: 193.
Shinojima A, Kawamura A, Mori R, Fujita K, Yuzawa M . Detection of morphologic alterations by spectral-domain optical coherence tomography before and after half-dose verteporfin photodynamic therapy in chronic central serous chorioretinopathy. Retina 2011; 31 (9): 1912.
Silva RM, Ruiz-Moreno JM, Gomez-Ulla F, Montero JA, Gregório T, Cachulo ML et al. Photodynamic therapy for chronic central serous chorioretinopathy: a 4-year follow-up study. Retina 2013; 33 (2): 309.
Smretschnig E, Ansari-Shahrezaei S, Hagen S, Glittenberg C, Krebs I, Binder S . Half-fluence photodynamic therapy in chronic central serous chorioretinopathy. Retina 2013; 33 (2): 316.
Smretschnig E, Ansari-Shahrezaei S, Moussa S, Glittenberg C, Krebs I, Binder S . Half-fluence photodynamic therapy in acute central serous chorioretinopathy. Retina 2012; 32 (10): 2014.
Taban M, Boyer DS, Thomas EL, Taban M . Chronic central serous chorioretinopathy: photodynamic therapy. Am JOphthalmol 2004; 137 (6): 1073–1080.
Tarantola RM, Law JC, Recchia FM, Sternberg P, Agarwal A . Photodynamic therapy as treatment of chronic idiopathic central serous chorioretinopathy. Lasers Surg Med 2008; 40 (10): 671–675.
Uetani R, Ito Y, Oiwa K, Ishikawa K, Terasaki H . Half-dose vs one-third-dose photodynamic therapy for chronic central serous chorioretinopathy. Eye 2012; 26 (5): 640.
Wali UK, Al-Kharousi N, Hamood H . Photodynamic therapy with verteporfin for chronic central serous choroidoretinopathy and idiopathic choroidal neovascularization-first report from the sultanate of oman. Oman Med J 2008; 23 (4): 282.
Yannuzzi LA, Slakter JS, Gross NE, Spaide RF, Costa DLL, Huang SJ et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina 2003; 23 (3): 288.
Yannuzzi LA . Type A behavior and central serous chorioretinopathy. Retina 2012; 32 (Suppl 1): 709.
Zhao M-W, Zhou P, Xiao H-X, Lv Y-S, Li C-A, Liu G-D et al. Photodynamic therapy for acute central serous chorioretinopathy: the safe effective lowest dose of verteporfin. Retina 2009; 29 (8): 1155.
Bae SH, Heo JW, Kim C, Kim TW, Lee JY, Song SJ et al. A randomized pilot study of low-fluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. Am J Ophthalmol 2011; 152 (5): 784–792 e2.
Chan WM, Lam DSC, Lai TYY, Tam BSM, Liu DTL, Chan CKM . Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol 2003; 87 (12): 1453.
Pryds A, Larsen M, Barbazetto I, Yannuzzi L . Central serous chorioretinopathy in young adults. Acta Ophthalmologica 2012; 90 (5): e404–e405.
Kim Young Seung, Y.H. L, Kim Hyoung Seok, Jin Sun Young, Lee Tae Gon . Comparison of therapeutic effect between half-energy photodynamic therapy and intravitreal bevacizumab injection in chronic central serous chorioretinopathy for 12 months. J Korean Ophthalmol Soc 2013; 54 (10): 1526–1533.
Arevalo JF, Espinoza JV . Single-session combined photodynamic therapy with verteporfin and intravitreal anti-vascular endothelial growth factor therapy for chronic central serous chorioretinopathy: a pilot study at 12-month follow-up. Graefes Arch Clin Exp Ophthalmol 2011; 249 (8): 1159–1166.
Fung AE, Palanki R, Bakri SJ, Depperschmidt E, Gibson A . Applying the CONSORT and STROBE statements to evaluate the reporting quality of neovascular age-related macular degeneration studies. Ophthalmol 2009; 116 (2): 286–296.
Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM . Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003; 23 (6): 752.
Maruko I, Iida T, Sugano Y, Furuta M, Sekiryu T . One-year choroidal thickness results after photodynamic therapy for central serous chorioretinopathy. Retina 2011; 31 (9)p. 1921.
Lee P, Kim K, Lee W . Severe choroidal ischemia following photodynamic therapy for pigment epithelial detachment and chronic central serous chorioretinopathy. Jpn J Ophthalmol 2009; 53 (1): 52–56.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Erikitola, O., Crosby-Nwaobi, R., Lotery, A. et al. Photodynamic therapy for central serous chorioretinopathy. Eye 28, 944–957 (2014). https://doi.org/10.1038/eye.2014.134
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.134
This article is cited by
-
Real-world practice patterns of eplerenone use for central serous chorioretinopathy
International Journal of Retina and Vitreous (2023)
-
Randomized controlled trials in central serous chorioretinopathy: A review
Eye (2023)
-
Predictive factors for outcomes of half-dose photodynamic therapy combined with aflibercept for pachychoroid neovasculopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Early versus delayed photodynamic therapy for chronic central serous chorioretinopathy
International Ophthalmology (2023)
-
Alternative management of central serous chorioretinopathy using intravitreal metoprolol
International Journal of Retina and Vitreous (2022)