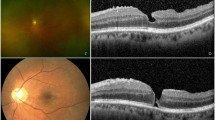
Abstract
Purpose
To evaluate the visual and anatomical results of surgery for macular hole-related retinal detachment (MHRD) after phacoemulsification cataract extraction.
Methods
Data for all patients who underwent surgery for MHRD after phacoemulsification cataract extraction from 1 December 1998 to 30 September 2008 in one hospital were evaluated. Patient characteristics, best-corrected visual acuity (VA) preoperatively and at last examination, surgical technique, anatomical success, and follow-up period were extracted and analysed statistically.
Results
A total of 13 625 eyes of 10 076 patients who had phacoemulsification cataract surgery were included. In the follow-up period, 10 cases of MHRD in nine patients were observed, of which seven eyes had high myopia. The mean axial length was 30.97±1.36 mm (29.19, 32.97) and mean myopia was−19.35±1.93 (−7.5,−3.5) dioptres. Overall anatomical success was achieved in 90% (9 out of 10 eyes). There was no statistically significant difference (P=0.240) between the logarithm of the MAR VA before the phacoemulsification cataract extraction and after MHRD surgical repair. VA increased in three eyes but decreased in the other seven after MHRD surgery.
Conclusions
As a primary procedure, vitreous surgery combined with other necessary adjunct procedures such as membrane peeling and retinal tamponade seems to be successful in achieving anatomical success. However, VA improvement is dependent on the type of macular lesion and not the surgical procedure.
Similar content being viewed by others
Introduction
A macular hole is a full-thickness defect of retinal tissue that involves the fovea and thereby affects central visual acuity (VA). Patterson et al1, 2 reported five eyes with macular hole formation in the early postoperative period after uncomplicated phacoemulsification. Patients usually complain of blurred vision, distortion, or metamorphopsia that persists despite normal recovery of the cornea and anterior segment after cataract surgery. Though it has been postulated that transmission of mechanical forces or vitreofoveal traction might be responsible, the exact aetiology of hole formation following cataract extraction is unclear. Central visual deterioration may be related to increasing and chronic subretinal fluid, cystoid retinal changes, or photoreceptor atrophy. Less commonly, loss of central and then peripheral vision is related to a progressive macular hole-related retinal detachment (MHRD). Our previous study reported a 0.073% incidence of MHRD after phacoemulsification and IOL implantation in an unselected group of patients.3 We also showed findings consistent with a previous report that MHRD occurred mainly in older women with myopia (>6D) and a posterior staphyloma.4, 5 MHRD is believed to arise from tangential traction on the retina by an epiretinal membrane or the inverse traction of the retina that cannot expand along with the posterior enlargement of the staphyloma.6, 7, 8, 9
As cases of cataract surgeries continue to increase, a higher incidence of MHRD is possible in the future. However, the visual and anatomical recoveries of surgery for MHRD after phacoemulsification have not been evaluated. In this study, we investigated these recoveries in an unselected group of patients.
Patients and methods
Between 1 December 1998 and 30 September 2008, phacoemulsification was performed on 13 625 eyes of 10 076 cataract patients at the Eye Hospital of Wenzhou Medical College. We excluded cases combined with other ocular procedures such as corneal grafts, glaucoma treatments, or posterior segment procedures. Other exclusion criteria included history of ocular trauma, retinal breaks in the operative eye, and development of endophthalmitis. The subjects were observed until the detection of an episode of MHRD, performance of any other intraocular procedure unrelated to MHRD repair, death, or end of study period.
The eye department where the study was conducted has standardised its surgical approach to cataract surgery. In almost all cases operated within the study period, phacoemulsification was performed using two-handed, downslope sculpting, nuclear cracking, and phacoaspiration of the fragments. Irrigation or aspiration was used to polish the capsular bag if necessary. At 9–74 months after phacoemulsification, a standard 3-port pars plana vitrectomy was performed using a contact lens viewing system for visualisation. Sclerotomies were placed 3.5 or 4 mm posterior to the limbus. Vitreoretinal procedures performed included vitrectomy, peeling of the posterior hyaloid membrane, epiretinal and internal membrane peeling, photocoagulation, cryotherapy, fluid–gas and oil–gas exchange, and gas or silicone oil injection. No case required posterior capsulotomy during surgery.
Patients were recorded by matching personal identification number with the diagnosis of MHRD. None of the included patients had been treated for MHRD in other eye clinics. The end-point of the study was surgical repair of MHRD performed in our hospital between the time of phacoemulsification and 30 September 2008. The endpoint was assessed by accessing the Thiseye System (Eye Hospital of Wenzhou Medical College, Wenzhou, China), which has recorded all diagnoses and surgical procedures on patients treated in our hospital since 1998.
For cases where differences were too small to define a proper significance level, the number of cases necessary to reach adequate significant levels was established by the log rank test. VA was measured using Snellen charts. The data was transformed into logarithm of the MAR (logMAR) units for statistical analysis. VA of finger counting, hand movements, and light perception was assigned to be equivalent to 1.6, 2.0, and 2.5 logMAR units, respectively. Average values are expressed as median (25th–75th percentile), and statistical significance was determined using Wilcoxon-Signed Rank Test. Statistical analysis was performed using SPSS (Statistical Package for Social Sciences Version 13.0, SPSS Inc., Chicago, IL, USA).
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
A total of 13 625 eyes of 10 076 patients who had phacoemulsification were included. There were significantly more women (5637) than men (4439) (P<0.001). The difference between the number of left eyes (49.18%) and right eyes (50.81%) was not significant (P=0.056). Most patients were between 65 and 79 years old; the average age was 67.22±15.10 years (range 42–105). The mean follow-up period was 48.22±31.10 (range 12–126) months. During the follow-up period, 10 cases of MHRD in nine patients were observed. The median interval between cataract surgery and development of a MHRD was 38.5 months (range 9–74 months). The mean age of subjects with MHRD was 60.8 years old (range 42–79). Seven of the ten eyes with MHRD had high myopia; the mean axial length was 30.97±1.36 mm (range 29.19–32.97) and the mean spherical equivalent was –19.35±1.93 (range –17.5 to –23.5) dioptres. The others were emmetropic eyes. One eye with MHRD occurred in a patient <49 years old; two eyes with MHRD were observed in patients 50–54, 55–59, and 70–74 years old, respectively; one eye with MHRD developed in patients 60–64, 65–69, and 75–79 years old, respectively. Two cases of MHRD occurred at 9 and 12 months; 3 cases at 22, 26, and 30 months; 5 cases at 51 (two eyes), 52, 58, and 74 months after cataract surgery, respectively. A summary of the recorded data for each patient is shown in Table 1.
In all cases, the macula had been confirmed to be normal by the examining surgeon after cataract removal. Metamorphopsia and central vision loss occurred an average of 38.5 months (range 9–74 months) after phacoemulsification. Among the 10 eyes with MHRD, 2 had an eccentric (can-opener) stage 2 full-thickness macular hole before MHRD surgery, 3 had a stage 3 hole, 4 had a stage 4 hole; and all contralateral eyes had a normal macula, except for one that had a stage 4 hole. All lesions demonstrated were confirmed by optical coherence tomography (OCT3, Carl Zeiss Meditec, Dublin, CA, USA) and there was no anterior–posterior vitreomacular traction (APVMT) in any of the 10 eyes. The macular holes ranged in size from one-fifth to half of a disc diameter. In 7 (70%) of the 10 eyes, the holes were irregular with a moth-eaten appearance; 3 eyes had a round hole. Two eyes had no peripheral holes, and the other eight had peripheral holes, which included all seven highly myopic eyes and one emmetropic eye. In highly myopic eyes, atrophic round holes usually occurred in areas involved in lattice and pigmentary degeneration in the peripheral fundus. In one emmetropic eye, only one hole arose in areas of paving-stone degeneration; there were one to four peripheral holes that ranged in size from one-fifth to two discs diameter. In all patients, peripheral retinal breaks were seen before MHRD surgery. An extensive RD that reached the ora serrata was observed in seven (70%) eyes with a posterior staphyloma involving the macular region. The other three (30%) eyes had no posterior staphyloma. In three eyes, the RD localised only within the staphylomatous cavity. Complete posterior vitreous detachment (PVD) was observed in eight eyes (80%), and no PVD was observed in two eyes (20%). No APVMT was found in any of the 10 eyes studied. An extensive RD developed in seven (70%) eyes with complete PVD and in two (20%) eyes without PVD.
All 10 eyes with MHRD had phacoemulsification, and 9 had the IOL implanted in the capsular bag. One IOL was placed in the ciliary sulcus due to concerns regarding posterior capsule break. An Nd:YAG capsulotomy had been performed in one eye (10%) 4 months before the diagnosis of MHRD. Following the first MHRD surgery, retinas were reattached in all 10 eyes, including 9 eyes after pars plana vitrectomy and 1 after posterior scleral reinforcement. Eight of the ten eyes were treated by pars plana vitrectomy and internal limiting membrane peeling with intravitreal silicone oil injection, whereas only two eyes were treated by intravitreal C3F8 injection with pars plana vitrectomy and internal limiting membrane peeling. Anatomical success was defined as total retinal attachment and the closure of the macular hole at the last follow-up examination. During the follow-up period, one eye that received pars plana vitrectomy relapsed. At the end of the follow-up period, all silicone oil was removed and total retinal reattachment was achieved in 9 of 10 eyes (Table 1).
Nine patients (10 eyes, 100%) had improvement in VA after phacoemulsification. The median (25th–75th percentile) logMAR VA increased from 1.1 (0.8, 1.6) before cataract surgery to 0.6 (0.175, 1.05) postoperatively (P=0.005). VA decreased to 1.8 (1.175, 2.0) (P=0.008) when MHRD occurred, and only one eye with MHRD had a logMAR VA that remained unchanged after cataract surgery. Despite the surgical repair of MHRD, there was no statistically significant difference between the preoperative MHRD logMAR VA and final logMAR VA (P=0.128). Five patients (50%) had improved VA, three patients (30%) remained the same, and two patients (20%) had decreased VA compared with the preoperative MHRD logMAR VA (Figure 1). There was no statistically significant difference (P=0.240) between the logMAR VA before phacoemulsification and after MHRD surgical repair. In fact, VA increased in three eyes and decreased in the other seven. Three eyes had the final logMAR VA of 1 (Snellen equivalent 6/60) or better; one eye had a logMAR VA of 0.3 (Snellen 6/12) or better; six eyes had a logMAR VA of 1.3 (Snellen equivalent 6/120) or worse.
Evolution of the different timepoints VA in 10 patients. The median (interquartile range) logMAR VA for patients before cataract surgery and postoperation were 1.1 (0.8, 1.6) and 0.6 (0.175, 1.05), respectively (P=0.005); the corresponding values for the MHRD occurred and the final follow-up were 1.8 (1.175, 2.0) and 1.3 (0.975, 2.0) (P=0.128). The length of each error bar corresponds to the interquartile range, with the upper boundary of the error bar representing the 75th and the lower boundary the 25th percentile. The point in the bar indicates the median value. CS, cataract surgery. MHRD, macular hole-related retinal detachment.
Discussion
In the past 20 years, phacoemulsification has become the preferred technique for cataract surgery in the developed world10, 11 and has enabled patients to achieve better functional results in a shorter time than with extracapsular techniques.12, 13 Furthermore, most studies have found the overall complication rate with phacoemulsification to be reduced.14, 15 Conversely, the rate of retinal detachment (RD) after cataract surgery has not been reduced significantly by the shift to phacoemulsification. RD is one of the most significant and severe events that can occur after all forms of cataract surgery, including phacoemulsification.16, 17, 18, 19, 20, 21, 22
Our previous study reported a 0.073% incidence of MHRD postphacoemulsification and IOL implantation in an unselected group of patients,3 which is much lower than the 0.7% incidence of non-MHRD after cataract surgery by phacoemulsification according to a previous report by Ramos et al.23 All patients had metamorphopsia or sudden decreased central vision 9–74 months out after cataract surgery. Although 8 eyes had peripheral holes, we had not found any peripheral holes by routine fundus examination after cataract surgery and considered them as atrophic retinal holes, which are usually asymptomatic and do not progress to retinal detachment. MHRD occurs as a separate entity in highly myopic eyes.24 Furthermore, nine patients (100%) had VA improvement after phacoemulsification. In 10 patients, the macular holes were seen after cataract surgery. All lesions demonstrated were confirmed by OCT and there was no APVMT in any of the 10 eyes. Thus, we considered the retinal detachments to have originated from macular holes and not peripheral holes.
In the current series, the median interval between cataract surgery and the development of a MHRD was 38.5 months. Although the difference in MHRD rates between men and women in our study was not statistically significant, 7 of the 10 MHRDs occurred in women who had high myopia, consistent with previous results of higher incidence in older women with highly myopic eyes and posterior staphylomas.5
High myopia, defined as a spherical equivalent of−6.0D or more and/or an axial length of at least 26.0 mm in our study, with macular holes may be complicated by retinal detachment, especially when associated with posterior staphyloma.4 However, the pathogenesis of this phenomenon remains controversial. We believe that in highly myopic eyes, the retina lacks sufficient elasticity to follow the posterior displacement of the scleral wall, which occurs as a result of continuous globe elongation and scleral ectasia. Eventually, a dehiscence occurs in its weakest part (i.e., the fovea or other weak points within the staphyloma) and RD ensues. Spontaneous PVD with apparent complete separation of the formed vitreous from the disc and retina in the posterior fundus is common in high myopia and were present in our patients. These findings support the dehiscence theory of macular hole formation and RD in highly myopic eyes.
The management for MHRD has been a challenge for decades.28 Before the introduction of pars plana vitrectomy, treatment consisted of macular buckle placement, in most cases combined with treatment of the macular hole by a method of retinopexy, including cryotherapy, diathermy,29 or photocoagulation.30, 31 However, many of these techniques had a considerable complication rate 31, 32 and were accompanied by extensive scarring in the macula with subsequent vision loss.9 In cases of high myopia with chorioretinal atrophy or a posterior staphyloma, treatment was less effective with earlier techniques because of the absence of retinal pigment epithelium (RPE) in areas of chorioretinal atrophy, leading to reduced natural retinal adhesion and inverse traction produced by posterior enlargement of the staphyloma overcoming retinal adhesion.27 In this situation, the retina would not have enough elasticity to follow the posterior displacement of the scleral wall.31
MHRD is usually treated by temporary intraocular gas tamponade with or without pars plana vitrectomy and laser photocoagulation around the macular hole.25 Vitrectomy with attempted removal of the epiretinal membrane or internal limiting membrane has been recently described.8 Pars plana vitrectomy techniques reduce the technical difficulties of conventional posterior scleral buckling surgery and result in better visual and anatomical outcomes.26 Treatment of MHRD with pars plana vitrectomy and air–fluid exchange would theoretically eliminate the need for buckling and photocoagulation around the macular hole by releasing vitreous traction on the macula. However, highly myopic eyes with large posterior staphylomas and extensive areas of atrophy did not respond well to such treatment, and long-term intraocular tamponade with silicone oil seems to have better long-term anatomical and functional results.27
Selected cases of MHRD in myopic eyes can be treated successfully by air or gas tamponade; however, this technique may be less effective in cases with chorioretinal atrophy and posterior staphyloma.9 Rosengren first reported intraocular tamponade using an air bubble combined with transscleral diathermy.33 Kreissig et al34 closed a posterior retinal hole with an expanding SF6 bubble without drainage of subretinal fluid. Using various perfluorocarbon gases, Lincoff et al reported successful reattachment in 15 of 16 retinal detachments caused by posterior retinal holes.35, 36 In Sobeilian's series,37 the primary surgical intervention in 12 eyes was intravitreal SF6 injection with an anatomical success rate of 41.7%. In our series, only two eyes were treated by intravitreal C3F8 injection with pars plana vitrectomy and internal limiting membrane peeling. Temporary macular hole tamponade with intravitreal gas is contraindicated in situations where intraocular proliferative tissue produces traction, leading to posterior retinal holes and detachments. These cases are most successfully repaired by release of intravitreal traction with pars plana vitrectomy. 38
Concomitant macular photocoagulation has not been uniformly used in previous studies. Bonnet and Semiglia39 observed late recurrence (between 7 and 78 months) after successful surgery for MHRD. Wolfensberger and Gonvers,40 who recognised the high rate of re-detachment if laser photocoagulation was not performed around the macular hole in eyes with chorioretinal atrophy or posterior staphyloma, used mild laser photocoagulation and silicone oil to obtain macular hole tamponade of longer duration. These investigators obtained a final successful retinal reattachment rate of 91% and removed silicone oil after a mean period of 3 months. Although Kreissig et al35 used argon or krypton laser coagulation to permanently secure the retina, others9, 26 did not use photocoagulation with equally successful results. Considering that 70% of the eyes in our series had posterior staphylomas and that laser photocoagulation has minimal or no effect in areas of severe RPE deficiency, we did not perform macular photocoagulation. In our series, for cases in which pars plana vitrectomy and silicone oil injection were performed as the first operation, anatomical attachment was achieved in 90% of the total cases without macular photocoagulation.
Another technique is peeling of residual vitreous cortex and internal limiting membrane, which is challenging in the presence of RD. The retina in highly myopic eyes is extremely thin and fragile and manipulation may lead to serious complications, even with the aid of perfluorocarbon liquids for retinal flattening. In highly myopic eyes with MHRD, the retina may be so fragile that enlargement of the hole may occur during internal limiting membrane peeling. Ishida et al reported successful retinal reattachment with epiretinal membrane removal in such cases.7 We also removed the residual cortical vitreous and internal limiting membrane peeling in nine eyes.
Anatomical success was obtained in 90% of the patients at the last follow-up examination, but VA improvement is mainly dependent on the individual macular lesion. Nine patients (100%) had VA improvement after phacoemulsification, and the VA decreased to 1.6 (P=0.008) when the MHRD occurred. Despite surgical repair of MHRD, there was no statistically significant difference (P=0.240) between the logMAR VA before the phacoemulsification and after the MHRD repair. In fact, VA increased in three eyes and decreased in the other seven. Improvement in VA after surgery is dependent on a number of factors, which include preoperative VA, intraoperative and postoperative complications, and the presence of a pre-existing ocular lesion, especially vitreoretinal diseases. Patients with more extensive macular pathology are less likely to experience significant improvement in vision, are more likely to have impaired macular function, and should therefore be given a guarded prognosis for cataract surgery. Interestingly, Sobeilian et al37 reported visual improvement in 78.6% of the patients with MHRD in highly myopic eyes after vitreous surgery. One of the explanations for this difference is that only three patients had a history of cataract extraction before the development of MHRD and 25 patients were phakic in Sobeilian's cases.37
We have performed a retrospective review of surgical repair of MHRD after phacoemulsification cataract extraction based on clinical records. We had a high rate of data retrieval and were able to collect information on potential risk factors such as axial length and surgical complications, validate laterality, and exclude a history of RD or vitreous surgery before cataract surgery.41, 42, 43 However, our study is subject to potential sources of error. We have assumed that the majority of patients that developed MHRD after cataract surgery attended the Eye Hospital at Wenzhou Medical College, a tertiary referral center for RD surgery, and that they were identified as cases. It is possible that some patients were treated for MHRD elsewhere, but the number of these patients would have to be substantial to generate sufficient bias to explain the large excess risk of MHRD found after posterior capsule tear. Other limitations of our study include the retrospective design, limited follow-up, and lack of a control group. Despite the large number of eyes in the study, the small number of events (MHRD) limits the significance of the statistical evaluation, especially in subgroups.
In summary, this study demonstrates that MHRD occurs after phacoemulsification cataract extraction and can be effectively repaired using conventional pars plana vitrectomy with a variety of adjuvant therapies. However, the improvement in VA is mainly dependent on the individual macular lesion, and MHRD-related surgical procedures have no direct relation with the visual improvement. The findings of this study highlight the need for full preoperative explanation of the long-term risk of MHRD, especially in the higher-risk subgroups of older women with high myopia.

References
Paques M, Massin P, Blain P, Duquesnoy AS, Gaudric A . Long-term incidence of reopening of macular holes. Ophthalmology 2000; 107: 760–766.
Patterson JA, Ezra E, Gregor ZJ . Acute full-thickness macular hole after uncomplicated phacoemulsification cataract surgery. Am J Ophthalmol 2001; 131: 799–800.
Li W, Zhao Y, Zheng Q, Wu R, Zheng J, Zheng B et al. Phacoemulsification complication. Ophthalmology 2010; 117 (1275): e1–e3.
Aaberg TM, Blair CJ, Gass JD . Macular holes. Am J Ophthalmol 1970; 69: 555–562.
Miller JH, Googe JM, Hoskins JC . Combined macular hole and cataract surgery. Am J Ophthalmol 1997; 123: 705–707.
Stirpe M, Michels RG . Retinal detachment in highly myopic eyes due to macular holes and epiretinal traction. Retina 1990; 10: 113–114.
Paques M, Massin P, Blain P, Duquesnoy AS, Gaudric A . Macular hole retinal detachment in highly myopic eyes: ultrastructure of surgically removed epiretinal membrane and clinicopathologic correlation. Retina 2000; 20: 176–183.
Oshima Y, Ikuno Y, Motokura M, Nakae K, Tano Y . Complete epiretinal membrane separation in highly myopic eyes with retinal detachment resulting from a macular hole. Am J Ophthalmol 1998; 126: 669–676.
Gonvers M, Machemer R . A new approach to treating retinal detachment with macular hole. Am J Ophthalmol 1982; 94: 468–472.
Apple DJ, Ram J, Foster A, Peng Q . Elimination of cataract blindness: a global perspective entering the new millenium. Surv Ophthalmol 2000; 45 (Suppl 1): S1–S196.
Elder M, Leaming D . The New Zealand cataract and refractive surgery survey 2001. Clin Exp Ophthalmol 2003; 31: 114–120.
Hoffman RS, Fine IH, Packer M . New phacoemulsification technology. Curr Opin Ophthalmol 2005; 16: 38–43.
Minassian DC, Rosen P, Dart JK, Reidy A, Desai P, Sidhu M . Extracapsular cataract extraction compared with small incision surgery by phacoemulsification: a randomised trial. Br J Ophthalmol 2001; 85: 822–829.
Seward HC, Dalton R, Davis A . Phacoemulsification during the learning curve: risk/benefit analysis. Eye 1993; 7 (Pt 1): 164–168.
Tarbet KJ, Mamalis N, Theurer J, Jones BD, Olson RJ . Complications and results of phacoemulsification performed by residents. J Cataract Refract Surg 1995; 21: 661–665.
Davison JA . Retinal tears and detachments after extracapsular cataract surgery. J Cataract Refract Surg 1988; 14: 624–632.
Nielsen NE, Naeser K . Epidemiology of retinal detachment following extracapsular cataract extraction: a follow-up study with an analysis of risk factors. J Cataract Refract Surg 1993; 19: 675–680.
Ninn-Pedersen K, Bauer B . Cataract patients in a defined Swedish population, 1986 to 1990. V. Postoperative retinal detachments. Arch Ophthalmol 1996; 114: 382–386.
Boberg-Ans G, Villumsen J, Henning V . Retinal detachment after phacoemulsification cataract extraction. J Cataract Refract Surg 2003; 29: 1333–1338.
Bhagwandien AC, Cheng YY, Wolfs RC, van Meurs JC, Luyten GP . Relationship between retinal detachment and biometry in 4262 cataractous eyes. Ophthalmology 2006; 113: 643–649.
Christensen U, Villumsen J . Prognosis of pseudophakic retinal detachment. J Cataract Refract Surg 2005; 31: 354–358.
Sheu SJ, Ger LP, Chen JF . Risk factors for retinal detachment after cataract surgery in southern Taiwan. J Chin Med Assoc 2005; 68: 321–326.
Ramos M, Kruger EF, Lashkari K. . Biostatistical analysis of pseudophakic and aphakic retinal detachments. Semin Ophthalmol 2002; 17: 206–213.
O'Driscoll AM, Goble RR, Kirkby GR . Vitrectomy for retinal detachments with both peripheral retinal breaks and macular holes. An assessment of outcome and the status of the macular hole. Retina 2001; 21: 221–225.
Blankenship GW, Ibanez-Langlois S . Treatment of myopic macular hole and detachment. Intravitreal gas exchange. Ophthalmology 1987; 94: 333–336.
Miyake Y . A simplified method of treating retinal detachment with macular hole. Long-term follow-up. Arch Ophthalmol 1986; 104: 1234–1236.
Gonvers M . Macular hole andretinal detachment. In: Blankenship GW, Binder S, Gonvers M, Stripe M (eds). Basic and Advanced Vitreous Surgery. Springer: Berlin-Heidelberg, 1986 pp 195–197.
Ripandelli G, Coppe AM, Fedeli R, Parisi V, D'Amico DJ, Stirpe M . Evaluation of primary surgical procedures for retinal detachment with macular hole in highly myopic eyes: a comparison [corrected] of vitrectomy vs posterior episcleral buckling surgery. Ophthalmology 2001; 108: 2258–2265 [Erratum appears in Ophthalmology 2002; 109: 222].
Adams ST . Retinal detachment due to macular and small posterior holes. Arch Ophthalmol 1961; 66: 528–533.
Howard GM, Campbell CJ . Surgical repair of retinal detachments caused by macular holes. Arch Ophthalmol 1969; 81: 317–321.
Siam AL . Management of central retinal detachment due to a macular hole. Br J Ophthalmol 1973; 57: 351–354.
Aaberg TM . Macular holes: a review. Surv Ophthalmol 1970; 15: 139–162.
Rosengren B . Results of treatment of detachment of the retina with diathermy and injection of air into the vitreous. Acta Ophthalmol 1938; 16: 573–579.
Kreissig I, Stanowsky A, Lincoff H, Richard G . The treatment of difficult retinal detachments with an expanding gas bubble without vitrectomy. Graefes Arch Clin Exp Ophthalmol 1986; 224: 51–54.
Lincoff H, Kreissig I, Brodie S, Wilcox L . Expanding gas bubbles for the repair of tears in the posterior pole. Graefes Arch Clin Exp Ophthalmol 1982; 219: 193–197.
Lincoff H, Coleman J, Kreissig I, Richard G, Chang S, Wilcox LM . The perfluorocarbon gases in the treatment of retinal detachment. Ophthalmology 1983; 90: 546–551.
Soheilian M, Ghaseminejad AK, Yazdani S, Ahmadieh H, Azarmina M, Dehghan MH et al. Surgical management of retinal detachment in highly myopic eyes with macular hole. Ophthalmic Surg Lasers Imaging 2007; 38: 15–22.
Blankenship GW . Posterior retinal holes secondary to diabetic retinopathy. Arch Ophthalmol 1983; 101: 885–887.
Bonnet M, Semiglia R . Late recurrences after successful surgery for retinal detachment with macular hole. Graefes Arch Clin Exp Ophthalmol 1993; 231: 347–350.
Wolfensberger TJ, Gonvers M . Long-term follow-up of retinal detachment due to macular hole in myopic eyes treated by temporary silicone oil tamponade and laser photocoagulation. Ophthalmology 1999; 106: 1786–1791.
Tielsch JM, Legro MW, Cassard SD, Schein OD, Javitt JC, Singer AE et al. Risk factors for retinal detachment after cataract surgery. A population-based case-control study. Ophthalmology 1996; 103: 1537–1545.
Ripandelli G, Scassa C, Parisi V, Gazzaniga D, D'Amico DJ, Stirpe M . Cataract surgery as a risk factor for retinal detachment in very highly myopic eyes. Ophthalmology 2003; 110: 2355–2361.
Inoue M, Shinoda K, Ishida S, Uchida A, Kurosaka D, Katsura H et al. Intraocular lens implantation after atopic cataract surgery decreases incidence of postoperative retinal detachment. Ophthalmology 2005; 112: 1719–1724.
Acknowledgements
We thank the surgeons who have managed the MHRD patients in this study. This study was supported by Wenzhou Medical College of Key Grants (QTJ05025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng, Q., Yang, S., Zhang, Y. et al. Vitreous surgery for macular hole-related retinal detachment after phacoemulsification cataract extraction: 10-year retrospective review. Eye 26, 1058–1064 (2012). https://doi.org/10.1038/eye.2012.87
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2012.87