Abstract
Purpose
The majority of patients with autosomal dominant optic atrophy (DOA) harbour pathogenic OPA1mutations. Although DOA is characterised by the preferential loss of retinal ganglion cells (RGCs), about 20% of patients with OPA1mutations will develop a more severe disease variant (DOA+), with additional neuromuscular features. In this prospective, observational case series, optical coherence tomography (OCT) was used to define the pattern of retinal nerve fibre layer (RNFL) loss in patients with both the pure and syndromal forms of DOA.
Methods
Forty patients with a molecular diagnosis of DOA due to OPA1mutations were prospectively recruited from our neuro-ophthalmology clinic: 26 patients with isolated optic atrophy and 14 patients manifesting DOA+ features. Peripapillary RNFL thickness was measured with the Fast RNFL (3.4) acquisition protocol on a Stratus OCT.
Results
There was a statistically significant reduction in average RNFL thickness in the OPA1group compared with normal controls (P<0.0001). The percentage decrease was greatest in the temporal quadrant (59.0%), followed by the inferior (49.6%), superior (41.8%), and nasal (25.9%) quadrants. Patients with DOA+ features had worse visual outcomes compared with patients with pure DOA. Except in the temporal quadrant, RNFL measurements were significantly thinner for the DOA+ group. There was an inverse correlation between average RNFL thickness and logarithm of the minimum angle of resolution (LogMAR) visual acuity (P<0.0001).
Conclusions
RGC loss in DOA is characterised by severe involvement of the temporal papillomacular bundle, with relative sparing of the nasal fibres. RNFL thinning is more pronounced in patients with DOA+ phenotypes.
Similar content being viewed by others
Introduction
Autosomal dominant optic atrophy (DOA, OMIM 605290) has an insidious onset in early childhood and it classically presents with bilateral, symmetrical, central visual loss and dyschromatopsia.1,2 About 60% of families harbour mutations in the OPA1 gene (3q28–q29), and over 200 pathogenic mutations have been reported with mutational hotspots in the GTPase and dynamin central domains.3,4 DOA is the most common inherited optic nerve disorder seen in the general population,5,6 and the majority of patients experience significant visual morbidity owing to progressive retinal ganglion cell (RGC) loss.7,8 Although the primary site of pathology is the RGC layer, up to 20% of patients with OPA1 mutations will also develop additional neuromuscular deficits, and we recently described the expanding clinical phenotypes associated with these complicated DOA+ variants.9,10,11 Sensorineural deafness was the most frequently observed extraocular feature, followed by chronic progressive external ophthalmoplegia, myopathy, ataxia, and peripheral neuropathy.9
The development of optical coherence tomography (OCT) has made it possible to quantify accurately the thickness of the retinal nerve fibre layer (RNFL) around the optic disc.12,13 This technology is currently being investigated in the management of glaucoma14,15 and other neuro-ophthalmological disorders such as multiple sclerosis16,17,18 and idiopathic intracranial hypertension.19 In this study, OCT imaging was used to compare the pattern of RGC loss in OPA1 patients with both the pure and syndromal forms of DOA, and to document the progression of RNFL thickness with disease duration.
Patients and methods
DOA cohort
We prospectively recruited 40 patients with confirmed pathogenic OPA1 mutations from our neuro-ophthalmology clinic: 26 patients with isolated optic atrophy and 14 patients manifesting DOA+ features (Table 1). The clinical and molecular descriptions of these patients have been detailed previously.5,9 Best-corrected visual acuity was measured using the Snellen chart and converted to logarithm of the minimum angle of resolution (LogMAR) decimal values for the purpose of statistical analysis.20 The peripapillary RNFL profile in our DOA cohort was compared with age-matched normal controls recruited among members of staff of our eye unit (N=15), and the normative database (N=328) included in the Stratus OCT operating software (Carl Zeiss Meditec, Dublin, CA, USA; http://www.zeiss.de/C12568E80025517D/EmbedTitelIntern/References_normatives/$File/czm_ndb_paper.pdf, accessed 18 September 2010). This study had the relevant institutional ethical approval and complied with the Declaration of Helsinki.
Optical coherence tomography
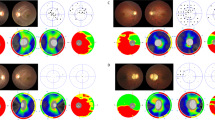
Peripapillary RNFL thickness was measured with the Fast RNFL (3.4) acquisition protocol on a Stratus OCT (Carl Zeiss Meditec), as described previously.21,22 Scans were repeated for each eye until proper centration of the optic disc was achieved within the 3.4 mm ring, with a signal strength ≥7. The analysis software automatically compares the measurements obtained with the appropriate normative range: (i) ≤29 years; (ii) 30–39 years; (iii) 40–49 years; (iv) 50–59 years; (v) 60–69 years; and (vi) ≥70 years. The RNFL parameters are colour-coded according to the normal distribution indices: (i) red <1%; (ii) yellow 1–5%; (iii) green 5–95%; and (iv) white >95% (Supplementary Figure 1).
Statistical analysis
Statistical analysis was performed using GraphPad v.4 statistical software (San Diego, CA, USA). The χ2-test and the independent sample t-test were used for group comparisons, as required. The relationship between RNFL thickness and LogMAR visual acuity were assessed with Spearman's rank correlation coefficient.
Results
Peripapillary RNFL profile among patients with OPA1 mutations
The majority of eyes from patients with OPA1 mutations had an average RNFL thickness <1% of the normal range (73/80, 91.3%), and this was also observed independently for the temporal (70/80, 87.5%), inferior (69/80, 86.3%), and superior (53/80, 66.3%) quadrants. For the nasal quadrant, only 13/80 (16.2%) eyes had an RNFL thickness <1%, compared with 44/80 (55.0%) eyes in the 5–95% range (Table 2). There was a significant difference in the distribution of RNFL thickness between quadrants (Supplementary Table 1), except for the comparison between the temporal and inferior quadrants (P=0.5868). To confirm these findings, peripapillary RNFL thickness in the OPA1 group (mean age=42.7 years, standard deviation (SD)=14.9 years, range=7.0–69.0 years) was compared with the measurements obtained from an independent cohort of 15 age-matched normal controls (mean age=40.3 years, SD=12.3 years, range=22.0–66.0 years, P=0.5745). OPA1 patients had a statistically significant reduction in mean RNFL thickness for all measurement parameters (Supplementary Figure 2, Table 3), and the percentage decrease was greater in the temporal quadrant (59.0%), followed by the inferior (49.6%), superior (41.8%), and nasal (25.9%) quadrants.
Comparison of RNFL thickness between pure DOA and DOA+ subgroups
There was no statistically significant difference in age between the pure DOA (mean=40.1 years, SD=15.7 years, range=7.0–69.0 years, N=26) and DOA+ subgroups (mean=47.6 years, SD=12.1 years, range=21.0–64.0 years, N=14, P=0.1295) (Supplementary Figure 3A). Patients with DOA+ features had significantly worse LogMAR visual acuities (mean=1.26, SD=0.85) compared with patients with pure DOA (mean=0.76, SD=0.54, P=0.0018) (Supplementary Figure 3B). Except for the temporal quadrant, RNFL measurements were significantly thinner in the DOA+ group (Figure 1, Table 4). There was a statistically significant inverse correlation between average RNFL thickness and LogMAR visual acuity (Figure 2), and this relationship was also significant for the inferior, superior, and nasal quadrants on subgroup analysis (Supplementary Table 2).
Discussion
The results of our study have confirmed the marked loss of RGC axons previously documented in post-mortem histopathological studies of two patients with DOA.23,24 OCT imaging performed on 40 patients with molecularly confirmed OPA1 mutations revealed a generalised decrease in peripapillary RNFL thickness, and a regional pattern was apparent, with the temporal quadrant being more severely affected and the nasal quadrant relatively spared. This preferential involvement of the papillomacular bundle is consistent with the characteristic wedge of temporal disc pallor observed clinically in DOA.5,25 It also supports earlier OCT reports of RGC loss in patients with OPA1 mutations.26,27,28 These studies, which only included patients with isolated optic atrophy, revealed a similar pattern of peripapillary RNFL thinning, with the temporal and nasal quadrants being the worst and least affected, respectively. Macular OCT measurements centred at the fovea showed no degeneration of the outer retina, and both the photoreceptor outer and inner segments were of normal thickness.27,28 Our larger case series included patients with both pure DOA and DOA+ phenotypes, allowing a more comprehensive subgroup analysis based on disease severity. Patients with DOA+ features had significantly worse visual acuities, and except for the temporal quadrant, RNFL thinning was more pronounced in this group compared with patients with only optic nerve involvement. There was also a strong correlation between RNFL thickness and LogMAR visual acuity. The greater deleterious consequences of some OPA1 mutations on RGC survival is therefore clearly linked to the development of multi-system organ involvement in DOA+. Further studies are warranted to define the pathophysiological pathways involved.9
It is important to stress that time-domain OCT has a minimum axial resolution of 30–40 μm,29 and it is entirely possible that RNFL thickness in the temporal quadrant was actually much lower than the values recorded. This could explain the lack of any significant difference in temporal RNFL thickness between patients with pure DOA and DOA+, and the absence of any significant correlation with LogMAR visual acuity for temporal quadrant measurements. Having already suffered the ‘greatest hit’ early in the disease process, the papillomacular bundle rapidly falls below the OCT detection threshold, but progression of RNFL thinning in the other quadrants is in keeping with the worsening of visual function and the development of DOA+ features.
The Opa1 protein is located within the mitochondrial inner membrane and it regulates several critical cellular functions related to the stability of the mitochondrial network, mitochondrial DNA (mtDNA) replication, and the activity of the respiratory chain complexes.30 Similar to Leber hereditary optic neuropathy (LHON), which is caused by primary mtDNA point mutations, RGC loss in DOA is ultimately due to disturbed mitochondrial oxidative function and apoptosis.1,2 It is therefore not surprising that both these mitochondrial optic neuropathies share striking similarities in RNFL abnormalities, with LHON carriers showing pathological thickening of the papillomacular bundle in the asymptomatic phase,21 and marked temporal thinning with relative nasal sparing in the atrophic phase of the disease.22
The assessment of peripapillary RNFL thickness with OCT is a useful tool now routinely available to clinicians when investigating optic nerve function. It is a practical, non-invasive procedure that can provide important structural information in borderline DOA cases and help direct subsequent investigations (Supplementary Figure 4). RNFL thinning in patients with OPA1 mutations is also a dynamic process and this provides an objective outcome measure for documenting disease progression in future treatment trials. Finally, the pattern of RGC loss observed in DOA reflects the intrinsic susceptibilities of different RGC populations to disturbed mitochondrial function. Understanding the key factors involved will be crucial in the development of novel neuroprotective strategies and in defining the therapeutic window for potential intervention.

References
Newman NJ, Biousse V . Hereditary optic neuropathies. Eye 2004; 18: 1144–1160.
Yu-Wai-Man P, Griffiths PG, Hudson G, Chinnery PF . Inherited mitochondrial optic neuropathies. J Med Genet 2009; 46: 145–158.
Ferre M, Amati-Bonneau P, Tourmen Y, Malthièry Y, Reynier P . eOPA1: an online database for OPA1 mutations. Hum Mutat 2005; 25: 423–428.
Ferre M, Bonneau D, Milea D, Chevrollier A, Verny C, Dollfus H et al. Molecular screening of 980 cases of suspected hereditary optic neuropathy with a report on 77 novel OPA1 mutations. Hum Mutat 2009; 30: E692–E705.
Yu-Wai-Man P, Griffiths PG, Burke A, Sellar PW, Clarke MP, Gnanaraj L et al. The prevalence and natural history of dominant optic atrophy due to OPA1 mutations. Ophthalmology 2010; 117: 1538.
Yu-Wai-Man P, Shankar SP, Biousse V, Miller NR, Bean LJ, Coffee B et al. Genetic screening for OPA1 and OPA3 mutations in patients with suspected inherited optic neuropathies. Ophthalmology 2010 (e-pub ahead of print 29 October 2010; doi:10.1016/j.ophtha.2010.07.029).
Votruba M, Moore AT, Bhattacharya SS . Clinical features, molecular genetics, and pathophysiology of dominant optic atrophy. J Med Genet 1998; 35: 793–800.
Cohn AC, Toomes C, Hewitt AW, Kearns LS, Inglehearn CF, Craig JE et al. The natural history of OPA1-related autosomal dominant optic atrophy. Br J Ophthalmol 2008; 24: 24.
Yu-Wai-Man P, Griffiths PG, Gorman GS, Lourenco CM, Wright AF, Auer-Grumbach M et al. Multi-system neurological disease is common in patients with OPA1 mutations. Brain 2010; 133: 771–786.
Amati-Bonneau P, Valentino ML, Reynier P, Gallardo ME, Bornstein B, Boissière A et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy plus phenotypes. Brain 2008; 131: 338–351.
Hudson G, Amati-Bonneau P, Blakely EL, Stewart JD, He L, Schaefer AM et al. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain 2008; 131: 329–337.
Subei AM, Eggenberger ER . Optical coherence tomography: another useful tool in a neuro-ophthalmologist's armamentarium. Curr Opin Ophthalmol 2009; 20: 462–466.
Sakata LM, DeLeon-Ortega J, Sakata V, Girkin CA . Optical coherence tomography of the retina and optic nerve—a review. Clin Exp Ophthalmol 2009; 37: 90–99.
Budenz DL, Fredette MJ, Feuer WJ, Anderson DR . Reproducibility of peripapillary retinal nerve fiber thickness measurements with stratus OCT in glaucomatous eyes. Ophthalmology 2008; 115: 661–666.
Medeiros FA, Zangwill LM, Alencar LM, Bowd C, Sample PA, Susanna Jr R et al. Detection of glaucoma progression with stratus OCT retinal nerve fiber layer, optic nerve head, and macular thickness measurements. Invest Ophthalmol Vis Sci 2009; 50: 5741–5748.
Henderson APD, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain 2008; 131: 277–287.
Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ . Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol 2008; 4: 664–675.
Talman LS, Bisker ER, Sackel DJ, Long Jr DA, Galetta KM, Ratchford JN et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol 2010; 67: 749–760.
Rebolleda G, Munoz-Negrete FJ . Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci 2009; 50: 5197–5200.
Holladay JT . Proper method for calculating average visual acuity. J Refract Surg 1997; 13: 388–391.
Savini G, Barboni P, Valentino ML, Montagna P, Cortelli P, De Negri AM et al. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber's hereditary optic neuropathy mutations. Ophthalmology 2005; 112: 127–131.
Barboni P, Savini G, Valentino ML, Montagna P, Cortelli P, De Negri AM et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber's hereditary optic neuropathy. Ophthalmology 2005; 112: 120–126.
Johnston PB, Gaster RN, Smith VC, Tripathi RC . Clinicopathologic study of autosomal dominant optic atrophy. Am J Ophthalmol 1979; 88: 868–875.
Kjer P, Jensen OA, Klinken L . Histopathology of eye, optic-nerve and brain in a case of dominant optic atrophy. Acta Ophthalmol 1983; 61: 300–312.
Votruba M, Thiselton D, Bhattacharya SS . Optic disc morphology of patients with OPA1 autosomal dominant optic atrophy. Br J Ophthalmol 2003; 87: 48–53.
Kim TW, Hwang JM . Stratus OCT in dominant optic atrophy—features differentiating it from glaucoma. J Glaucoma 2007; 16: 655–658.
Ito Y, Nakamura M, Yamakoshi T, Lin J, Yatsuya H, Terasaki H . Reduction of inner retinal thickness in patients with autosomal dominant optic atrophy associated with OPA1 mutations. Invest Ophthalmol Vis Sci 2007; 48: 4079–4086.
Milea D, Sander B, Wegener M, Jensen H, Kjer B, Jørgensen TM et al. Axonal loss occurs early in dominant optic atrophy. Acta Ophthalmol 2010; 88: 342–346.
Chauhan DS, Marshall J . The interpretation of optical coherence tomography images of the retina. Invest Ophthalmol Vis Sci 1999; 40: 2332–2342.
Lenaers G, Reynier P, ElAchouri G, Soukkarieh C, Olichon A, Belenguer P et al. OPA1 functions in mitochondria and dysfunctions in optic nerve. Int J Biochem Cell Biol 2009; 41: 1866–1874.
Acknowledgements
PYWM is a Medical Research Council (MRC, UK) Clinical Research Fellow in Neuro-Ophthalmology and PFC is a Wellcome Trust Senior Fellow in Clinical Science. PFC also receives funding from the Parkinson's Disease Society (UK), the MRC Translational Muscle Centre, and the UK NIHR Biomedical Research Centre in Ageing and Age Related Disease.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Eye website
Rights and permissions
About this article
Cite this article
Yu-Wai-Man, P., Bailie, M., Atawan, A. et al. Pattern of retinal ganglion cell loss in dominant optic atrophy due to OPA1 mutations. Eye 25, 596–602 (2011). https://doi.org/10.1038/eye.2011.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.2