Abstract
Purpose
The aim of this study was to test the correlation between Fourier-domain (FD) optical coherence tomography (OCT) macular and retinal nerve fibre layer (RNFL) thickness and visual field (VF) loss on standard automated perimetry (SAP) in chiasmal compression.
Methods
A total of 35 eyes with permanent temporal VF defects and 35 controls underwent SAP and FD-OCT (3D OCT-1000; Topcon Corp.) examinations. Macular thickness measurements were averaged for the central area and for each quadrant and half of that area, whereas RNFL thickness was determined for six sectors around the optic disc. VF loss was estimated in six sectors of the VF and in the central 16 test points in the VF. The correlation between VF loss and OCT measurements was tested with Spearman's correlation coefficients and with linear regression analysis.
Results
Macular and RNFL thickness parameters correlated strongly with SAP VF loss. Correlations were generally stronger between VF loss and quadrantic or hemianopic macular thickness than with sectoral RNFL thickness. For the macular parameters, we observed the strongest correlation between macular thickness in the inferonasal quadrant and VF loss in the superior temporal central quadrant (ρ=0.78; P<0.001) whereas for the RNFL parameters the strongest correlation was observed between the superonasal optic disc sector and the central temporal VF defect (ρ=0.60; P<0.001).
Conclusion
Although FD-OCT RNFL and macular thickness measurements were both correlated with VF loss, the correlation was stronger with quadrantic macular than with RNFL thickness measurements in patients with temporal hemianopia. Such measurements could potentially be used to quantify neuronal loss in patients with chiasmal compression.
Similar content being viewed by others
Introduction
The evaluation of anterior visual pathway diseases involves both psychophysical examinations, such as visual acuity and visual field (VF) tests, and structural assessments of the optic disc, peripapillary retinal nerve fibre layer (RNFL) and retinal ganglion cell integrity. Studies investigating the relationship between these parameters are of great importance not only to provide complementary data on the extent of neural damage in patients at risk of irreversible VF loss, but even more importantly to help estimate the chances of reversing visual loss in conditions such as demyelinating or compressive optic neuropathy.1, 2, 3 However, most structure–function relationship studies have focused on patients with glaucoma,4, 5, 6, 7 and conclusions have not been directly extrapolatable to other optic pathway conditions.
The changes in the structural appearance of the optic nerve head (ONH) and RNFL that accompany the development of VF loss in patients with permanent temporal hemianopia from chiasmal compression represent an important model for assessing the diagnostic performance of different instruments for measuring retinal neural structures. Patients with chiasmal lesions affecting the crossed nerve fibres and causing bitemporal hemianopia with preserved nasal field generally present clearly perceptible changes in the peripapillary area. In these cases, RNFL loss occurs predominantly on the nasal and temporal side of the optic disc, a pattern identified on ophthalmoscopy as band atrophy (BA) of the RNFL,8, 9, 10, 11 and there is preservation of the uncrossed nerve fibres, which originate in the temporal hemiretina and penetrate the optic nerve through the superior and inferior arcuate fibre bundles. Using time-domain (TD) optical coherence tomography (OCT), Danesh-Meyer et al1 studied the spatial relationship between RNFL thickness measured with TD-OCT and VF sensitivity measured with standard automated perimetry (SAP) in patients with chiasmal compression and found close correlations particularly with the temporal sector of the optic disc. Macular thickness was not investigated.
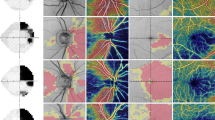
So far, most studies on structure–function relationships have used RNFL thickness measurements to evaluate axonal damage. However, several studies have indicated that macular thickness measurements may also be used as an indicator of neural loss. Because the ganglion cell layer accounts for up to 40% of the thickness in the macular area, estimates of macular thickness can be used to investigate possible ganglion cell loss. In fact, previous OCT-based studies have shown that the macula is significantly thinner in eyes with glaucoma12, 13, 14 and BA than in healthy eyes.15 Similarly, TD-OCT macular thickness measurements (Stratus-OCT; Carl Zeiss Meditec, Dublin, CA, USA) have shown a close correlation between combined nasal average macular thickness (average of the inner and outer areas of the macular map) and temporal VF loss in patients with chiasmal lesions. However, the macular data (obtained from six radial Stratus-OCT scans) could not be matched directly with the corresponding areas of VF defect. When investigating patients with temporal VF defects that respect the vertical meridian, it seemed likely that a division of the macular area in quadrants would provide a stronger correlation with VF loss than OCT data organized according to a circular map with values presented in sectors that do not respect the vertical meridian (Figure 1). It also seemed likely to have the potential for providing better correlations with VF loss than assessments of RNFL around the ONH, because most sectors of the disc receive nerve fibres from ganglion cells corresponding to both the nasal and the temporal hemifield.1, 4, 16
Visual field and fundus photograph of eye with complete temporal hemianopia (upper row) or superior temporal quadrantanopia (second row), showing schematic demarcation between relatively preserved ganglion cells (lighter) and atrophic ganglion cells (darker), corresponding to the visual field defect. Note the greater appropriateness of the macular thickness measurements data division obtained with Fourier-domain (middle) compared to time-domain optical coherence tomography (right).
Recent innovations in OCT technology include three-dimensional high-resolution scanning with Fourier-domain (FD) detection as an improvement over conventional TD-OCT technology.17 Cross-sectional retinal images with an axial resolution up to 5 times higher and imaging speeds approximately 60 times greater than conventional OCT have been acquired in vivo.18, 19 This permits high-density raster scanning of retinal tissue while minimizing eye motion artefacts.20 A recent study comparing FD and TD-OCT thickness measurements in patients with BA of the optic nerve, using similar measuring protocols, indicates that both technologies are able to differentiate BA from normal eyes and that FD-OCT has the potential to generate more information than TD-OCT scanning.21 However, another potential advantage for three-dimensional high-resolution scanning when investigating structure–function correlation in patients with chiasmal or retrochiasmal (optic tract and geniculate body) VF defects is that macular thickness data can be segmented by quadrant and can be directly correlated with VF in each quadrant, a subject which has not previously been investigated.
The purpose of this study was therefore to test the correlation between FD-OCT macular thickness measurements, measured globally and in quadrants, and VF loss on SAP in eyes with permanent temporal VF defect from chiasmal compression. In addition, we have investigated the spatial correlation between SAP measured VF loss and FD-OCT measured RNFL thickness in different optic nerve segments in the same sample of patients.
Materials and methods
Subjects
A total of 35 eyes from 35 patients (20 men) with temporal hemianopia from chiasmal compression and 35 eyes from 35 controls (14 men) were recruited for examination at the Division of Ophthalmology of the University of São Paulo Medical School. All patients had been submitted to previous treatment of the suprasellar lesion and had stable VF defects for at least 1 year before study entry. Thirty patients had pituitary adenoma, three had craniopharyngioma, and two had suprasellar meningioma. The study adhered to the tenets of the Declaration of Helsinki Principles and informed consent was obtained from all participants. Approval from the institutional review board ethics committee was obtained for the study.
The inclusion criteria for the study were best corrected VA of 20/30 or better in the study eye; spherical refraction within ±5 D and cylinder refraction within ±4 D; intraocular pressure <22 mm Hg; and reliable VF. Patients with a history of intraocular diseases or with clinical signs of glaucoma or optic disc anomaly were excluded.
Patients with BA were required to have complete or partial temporal hemianopia and a nasal hemifield within normal limits. The minimum criteria for defining a VF as abnormal was the presence of at least two non-edge contiguous test points, not including those directly above and below the blind spot, with a pattern deviation (PD plot) of one point with P<0.5% and one point with P<2%. On SAP, a normal nasal hemifield was defined as the absence of any cluster of at least three points with P<5% on the PD plot. Only one eye of each patient was selected for analysis.
The control group consisted of normal healthy volunteers recruited from among the hospital staff. All normal subjects had normal ophthalmic findings and normal VF. A normal VF was defined as a pattern standard deviation within the 95% confidence limits and a Glaucoma Hemifield Test result within normal limits. One eye of each healthy subject was included for analysis.
High-resolution FD-OCT scanning
Subjects underwent FD-OCT raster scanning of the ONH region and macular area without dilating the pupil, using commercially available equipment (3D OCT-1000; Topcon Corp., Tokyo, Japan). The instrument uses a near-infrared, low-coherence superluminescent diode light source with a 50 nm bandwidth and a centre wavelength of 840 nm, corresponding to an axial resolution of 6 μm and a lateral resolution of 10 μm.
The scanning protocol used in this study involved the acquisition of a set of three high-definition OCT images of the ONH and macula in a raster pattern covering a 6 × 6 mm (Figure 2) area with a scan density of 512 × 128 pixels in ∼3.5 s (18 000 A scans per second). Criteria for acceptable 3D OCT-1000 fundus images included the following: (1) no large eye movements, defined as an abrupt shift completely disconnecting a large retinal vessel; (2) consistent signal intensity level across the scan, and (3) no black bands (caused by blinking) throughout the examination.
Top row: Demarcation of areas in the macula (left) and optic nerve (right) scanned by Fourier-Domain (FD) optical coherence tomography (OCT). Middle row: Schematic representation of a macular thickness map (left) and retinal nerve fibre layer thickness (right) of a normal individual. Bottom row: (Left) Demarcation of points read on 24-2 standard automated perimetry. The 16 points contained in the central square correspond to the area covered by FD-OCT scanning. (Right) division of the 24 visual field (VF) test points and the optic nerve head into sectors, derived from a published optic disc visual field map.16 The vertical dotted line indicates where field sector 6 was divided along the vertical meridian.
Peripapillary RNFL thickness and macular RNFL thickness parameters were automatically calculated with the software provided by the manufacturer. Macular thickness measurements were registered according to an overlaid OCT-generated checkerboard with 36 checks. The macular thickness parameters were subsequently averaged separately for each of the four quadrants (nine checks per quadrant) of the macular area: superotemporal, inferotemporal, superonasal, and inferonasal (Figure 2). The average thickness of the macula and of each hemiretina (18 nasal and 18 temporal checks) was also calculated. In addition, thickness measurements were taken using a circular (∅=3.4 mm) RNFL peripapillary map drawn around the ONH, when taking measurements corresponding to average thickness (360°), and the following ONH sectors: temporal (310°–41°), superotemporal (41°–80°), superonasal (80°–120°), nasal (121°–230°), inferonasal (231°–270°) and inferotemporal (271°–310°) (Figure 2).
Standard automated perimetry
SAP was conducted with a Humphrey Field Analyzer (Carl Zeiss Meditec) using the Swedish Interactive Threshold Algorithm (SITA standard 24.2 program) and a Goldmann size III stimulus on a 31.5-apostilb background. Perimetry was performed on the same day as OCT testing. All VFs included in the study had reliability indices of less than 30% false positives, false negatives, or fixation losses.
The severity of VF defects in patients with BA was determined by evaluating VF sensitivity (VFS) loss from the 16 central points of the VF, an area roughly equivalent to the area covered by 3D OCT-1000 raster scanning in the macular area. The values of the total deviation plot of the SAP 24-2 test covering these 16 test points (18° × 18°) were averaged globally to calculate the central mean deviation of each quadrant (superotemporal, inferotemporal, superonasal, and inferonasal) and of each half (central nasal and central temporal). Thus, each quadrant represented the average deviation of four VF test points, and each half the average deviation of eight VF test points (Figure 2, bottom left).
VFS loss was also determined for six VF sectors according to a previously published optic disc-VF map.16 These six sectors correspond to the inferonasal, inferotemporal, temporal, superotemporal, superonasal, and nasal optic disc sector. The temporal sector was further subdivided by a line along the vertical meridian, and the four points located temporally to the vertical meridian were averaged and labelled as central temporal VF (CTVF) (Figure 2, bottom right).
Deviation from normal in the total deviation plot at each test location was measured in decibels. Unlogged 1/Lambert (1/L) values were calculated for each test location by dividing the decibel readings by 10 and then unlogging the quotient.
Data analysis and statistics
Spearman's ranked correlation coefficients (ρ) were used to assess the strength of possible associations between retinal thickness and VFS loss. Subsequently, the relationship between FD-OCT measurements and VF loss expressed in 1/L scale was described with linear regression analysis based on the model proposed by Hood et al.22 VF data were treated as dependent variables and FD-OCT measurements as independent variables in all regressions. Macular and RNFL thickness values of eyes with BA and normal controls were compared using Student's t-tests. P-values of less than 0.05 were considered statistically significant.
Results
A total of 35 eyes with temporal hemianopia and 35 control eyes were studied. The mean age±standard deviation (SD) was 47.23±12.70 years (range 22–71) in BA patients, and 43.00±12.62 years (range 27–69) in normal subjects (P=0.17; Student's unpaired t-test). The fundoscopic examination revealed signs of BA of the optic disc and RNFL in all 35 eyes.
The mean macular thickness was 216.38 μm in eyes with BA and 239.78 μm in control eyes (P<0.001). In eyes with BA, the mean quadrantic macular thickness was 214.72±11.36, 213.03±9.92, 220.70±11.57, and 217.47±13.18 μm in the superotemporal, inferotemporal, superonasal, and inferonasal macular quadrants, respectively. The corresponding figures for the control eyes were 227.48±12.86, 226.02±15.10, 255.09±15.92, and 250.44±16.16 μm, respectively. The average RNFL thickness was 57.37 μm in eyes with BA and 89.75 μm in control eyes (P<0.001). In eyes with BA, the RNFL thickness was 88.60±22.92, 63.97±27.68, 96.05±24.28, 79.54±19.16, 38.23±20.55, and 36.89±15.42 μm in the superotemporal, superonasal, inferotemporal, inferonasal, nasal, and temporal optic disc sectors, respectively. The corresponding figures for the control eyes were 105.11±18.97, 101.62±14.23, 126.71±20.34, 121.51±16.80, 74.26±12.70, and 66.03±10.11 μm, respectively. All macular and RNFL thickness parameters were significantly lower for eyes with BA than for normal eyes (P<0.001).
Table 1 shows the VF data of eyes with BA. The mean±SD of SAP mean deviation was −10.19±4.87 dB. Deviation from normal, corresponding to the 16 central points of the VF, and data from each VF sector are also presented in Table 2. Within the area covered by the 16 central points, the temporal VF sectors were more significantly depressed and more frequently outside normal limits than the nasal sectors in every instance (P<0.001). The most significantly depressed VF parameters were the temporal half and the superotemporal quadrant. Table 1 also presents data from VF areas divided according to the VF map designed by Garway-Heath et al.16 The areas with the greatest VFS loss on this map were sector 1 (the four most temporal points of the VF) with a loss of 23 dB (corresponding to the nasal disc sector) and the temporal part of sector 6 (corresponding to the papillomacular bundle) with a loss of 19.77 dB.
Table 2 shows the correlations between macular thickness parameters and the severity of VF loss based on the 16 central points of the VF (in decibels and 1/L units). Statistically significant correlations were found for most parameters. The most significant correlations were found between the inferonasal macular quadrant and the superotemporal central VF quadrant (ρ=0.78, P<0.001), between the nasal half macular average and the superotemporal central VF quadrant (ρ=0.75, P<0.001), and between the superonasal macular quadrant and the inferotemporal central VF quadrant (ρ=0.65, P<0.001). Correlations were the same whether VFS loss was assessed in dB or in 1/L.
Table 3 shows associations between RNFL thickness and VF parameters based on different VF sectors according to a predefined map. The three most significant correlations were between the superonasal disc sector and the CTVF defect (CTVFD) (ρ=0.60, P<0.001), the inferotemporal disc sector and VF sector 6 (ρ=0.57, P<0.001), and the average RNFL thickness and the CTVFD (ρ=0.53, P<0.001), when VF loss was assessed in 1/L units.
Figure 3 shows the results from the linear regression analysis of the best-performing macular or RNFL thickness measurements and VF loss (in 1/L units). For the macular measurements, we observed the greatest sectoral R2 between the CTVF loss and the nasal half macular thickness (R2=45%, P<0.001), between the superotemporal quadrant VF loss and the inferonasal quadrant macular thickness (R2=45%, P<0.001), and between the inferotemporal quadrant VF defect and the superonasal quadrant macular thickness (R2=37%, P<0.001). For the RNFL thickness measurements, we observed the greatest R2 between the CTVFD and the RNFL average thickness or the superonasal optic disc sector (R2=41%, P<0.001) and between VF defect in sector 6 and the inferotemporal optic disc sector (R2=40%, P<0.001).
Conclusion
Because Zeimer et al 23 proposed to use macular thickness as an indicator of glaucoma severity, several studies have investigated this idea using technologies to measure retinal thickness such as OCT.12, 13, 14, 24, 25 Such studies were conducted based on the theory that the macular thickness might be superior to circumpapillary RNFL for quantification of axonal loss, because retinal ganglion cell bodies are 10–20 times the diameter of their axons and because the retinal ganglion cell is more than one cell thick in the macula.26 However, despite the confirmation that macular thickness is in fact reduced in patients with glaucoma, several studies have reported circumpapillary RNFL thickness to be a more sensitive detector of glaucoma than macular thickness.13, 27, 28 This may in part be due to limitations inherent in the OCT software that is restricted to analysing macular data for the entire retina, leading to reduced specificity. In this context it should be recalled that glaucoma and other optic neuropathies affect only the inner retinal layers whereas the outer layers merely increase measurement variability.29 Conversely, technological improvements in image resolution have made segmentation and measurement of individual retinal layers possible, and a macular segmentation algorithm for OCT data has been developed by Ishikawa et al.26 Using such algorithm in TD-OCT data, the authors have found macular thickness measurements to be as reliable as RNFL thickness measurements for detecting glaucoma, a finding recently confirmed by Tan et al.29 The possibility of macular segmentation and the high macular image resolution provided by FD-OCT are likely to increase interest in the use of macular thickness measurements for quantifying neural loss in glaucoma and other anterior optic pathway diseases.
In this study, macular thickness and RNFL thickness were both significantly smaller in eyes with BA of the optic nerve than in healthy control eyes, which is in agreement with several previous studies. However, more than just testing the diagnostic ability of FD-OCT in patients with BA, this study evaluates the usefulness of this new technology in the assessment of structure–function relationships in patients with temporal field defects by measuring macular thickness in quadrants. The study was specifically designed to investigate the relationship between VF defects and RNFL or macular thickness measurements divided in quadrants and in halves. Our findings indicate that although both RNFL and macular thickness parameters performed well, macular thickness parameters may have a greater potential than RNFL thickness parameters for assessing structure–function relationships in chiasmal disease. Although Spearman's correlation between nasal macular parameters and VFS ranged from 0.65 to 0.78, the correlation between the best-performing sectoral RNFL thickness parameters and VFS was 0.60 at most. The former association was also stronger when using linear regression analysis (Figure 3).
The close structure–function relationship observed between macular parameters and optic disc sectors is most likely due to the anatomical disposition of the RNFL on the disc. With the exception of the nasal area of the disc (sector 1), all other sectors receive retinal nerve fibres from both the nasal and the temporal hemiretina. The result is a lack of specificity between VF loss and optic disc sectors in patients with lesions of the optic chiasm. For example, when a VF defect affecting the superotemporal quadrant is present, it results in RNFL loss in the nasal, temporal, and inferior regions of the optic disc. Because the inferior region of the optic disc also receives nerve fibres from the unaffected nasal VF, there is presumably a lack of specificity in the structure–function relationship. Conversely, with macular thickness measurements a superotemporal VF defect may be related to reduced macular thickness below and nasally to the fovea, with a direct correspondence to the quadrantic VF defect. The advantage of the technique would be even greater in patients with VF defect affecting the superior and inferior temporal quadrants. On the basis of the distribution of the RNFL on the optic disc,16 such patients may be expected to have RNFL loss in every quadrant of the optic disc, with a resultant lack of specificity in the structure–function relationship, whereas macular thickness reduction would be observed almost exclusively nasally to the fovea, probably with a much higher specificity. The findings of this study support this hypothesis because the closest structure–function relationships observed were in fact those between nasal macular thickness parameters and the temporal VF defect.
The close structure–function relationship is probably the result of the division of the macular area in quadrants. Such a division was not possible with the earlier TD-OCT technology because macular thickness measurements were obtained from six radial scans precluding a good working division of macular data in quadrants. The current OCT technology made it possible to divide the macular area in quadrants and evaluate the relationship between macular thickness parameters and VF defects (Figure 1). Recent studies have shown that the processing of macular thickness measurements in segments can improve diagnostic ability significantly compared to RNFL thickness measurements. The increased spatial resolution obtained with FD-OCT will presumably make macular segmentation easier and may lead to a comparative advantage for macular measurements over RNFL thickness measurements in the quantification of neural loss in patients with chiasmal or optic tract lesions. Future studies using macular segmentation in sectors and quadrants will probably be very useful in diagnosing and managing patients of this type.
This study indicates that FD-OCT-assessed structure–function relationships are adequate for assessing the correspondence between VF loss and neural loss based on either RNFL or macular thickness measurements. Also, in our study, as well as in previous ones, only the current macular thickness parameters currently provided by the standard 3D OCT printout were investigated. It is possible that advances in the software designed to extract data from the macular area will improve detection of retinal ganglion cell loss in the posterior pole in patients with BA of the optic nerve and related conditions.

References
Danesh-Meyer HV, Carroll SC, Foroozan R, Savino PJ, Fan J, Jiang Y et al. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Invest Ophthalmol Vis Sci 2006; 47: 4827–4835.
Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD . In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci 2008; 49: 1879–1885.
Jacob M, Raverot G, Jouanneau E, Borson-Chazot F, Perrin G, Rabilloud M et al. Predicting visual outcome after treatment of pituitary adenomas with optical coherence tomography. Am J Ophthalmol 2009; 147: 64.e2–70.e2.
Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA . Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci 2002; 43: 2213–2220.
Bowd C, Zangwill LM, Medeiros FA, Tavares IM, Hoffmann EM, Bourne RR et al. Structure-function relationships using confocal scanning laser ophthalmoscopy, optical coherence tomography, and scanning laser polarimetry. Invest Ophthalmol Vis Sci 2006; 47: 2889–2895.
El Beltagi TA, Bowd C, Boden C, Amini P, Sample PA, Zangwill LM et al. Retinal nerve fiber layer thickness measured with optical coherence tomography is related to visual function in glaucomatous eyes. Ophthalmology 2003; 110: 2185–2191.
Leung CK, Chan WM, Chong KK, Yung WH, Tang KT, Woo J et al. Comparative study of retinal nerve fiber layer measurement by StratusOCT and GDx VCC, I: correlation analysis in glaucoma. Invest Ophthalmol Vis Sci 2005; 46: 3214–3220.
Unsold R, Hoyt WF . Band atrophy of the optic nerve. The histology of temporal hemianopsia. Arch Ophthalmol 1980; 98: 1637–1638.
Monteiro ML, Medeiros FA, Ostroscki MR . Quantitative analysis of axonal loss in band atrophy of the optic nerve using scanning laser polarimetry. Br J Ophthalmol 2003; 87: 32–37.
Monteiro ML, Leal BC, Rosa AA, Bronstein MD . Optical coherence tomography analysis of axonal loss in band atrophy of the optic nerve. Br J Ophthalmol 2004; 88: 896–899.
Monteiro ML, Leal BC, Moura FC, Vessani RM, Medeiros FA . Comparison of retinal nerve fibre layer measurements using optical coherence tomography versions 1 and 3 in eyes with band atrophy of the optic nerve and normal controls. Eye 2007; 21: 16–22.
Greenfield DS, Bagga H, Knighton RW . Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch Ophthalmol 2003; 121: 41–46.
Guedes V, Schuman JS, Hertzmark E, Wollstein G, Correnti A, Mancini R et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology 2003; 110: 177–189.
Lederer DE, Schuman JS, Hertzmark E, Heltzer J, Velazques LJ, Fujimoto JG et al. Analysis of macular volume in normal and glaucomatous eyes using optical coherence tomography. Am J Ophthalmol 2003; 135: 838–843.
Moura FC, Medeiros FA, Monteiro ML . Evaluation of macular thickness measurements for detection of band atrophy of the optic nerve using optical coherence tomography. Ophthalmology 2007; 114: 175–181.
Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA . Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology 2000; 107: 1809–1815.
Wojtkowski M, Srinivasan V, Fujimoto JG, Ko T, Schuman JS, Kowalczyk A et al. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 2005; 112: 1734–1746.
Wojtkowski M, Srinivasan V, Ko T, Fujimoto J, Duker J, Schuman J et al. High speed, ultrahigh resolution retinal imaging using spectral/Fourier domain OCT. Conf Lasers Electrooptics 2005; 3: 2058–2060.
Wojtkowski M, Srinivasan VJ, Ko TH, Fujimoto J, Kowalczyk A, Duker J . Ultrahigh-resolution, high-speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Opt Express 2004; 12: 2404–2422.
Gabriele ML, Ishikawa H, Wollstein G, Bilonick RA, Kagemann L, Wojtkowski M et al. Peripapillary nerve fiber layer thickness profile determined with high speed, ultrahigh resolution optical coherence tomography high-density scanning. Invest Ophthalmol Vis Sci 2007; 48: 3154–3160.
Costa-Cunha LV, Cunha LP, Malta RF, Monteiro ML . Comparison of Fourier-domain and time-domain optical coherence tomography in the detection of band atrophy of the optic nerve. Am J Ophthalmol 2009; 147: 56.e2–63.e2.
Hood DC, Anderson S, Rouleau J, Wenick AS, Grover LK, Behrens MM et al. Retinal nerve fiber structure versus visual field function in patients with ischemic optic neuropathy. A test of a linear model. Ophthalmology 2008; 115: 904–910.
Zeimer R, Shahidi M, Mori M, Zou S, Asrani S . A new method for rapid mapping of the retinal thickness at the posterior pole. Invest Ophthalmol Vis Sci 1996; 37: 1994–2001.
Wollstein G, Schuman JS, Price LL, Aydin A, Beaton SA, Stark PC et al. Optical coherence tomography (OCT) macular and peripapillary retinal nerve fiber layer measurements and automated visual fields. Am J Ophthalmol 2004; 138: 218–225.
Giovannini A, Amato G, Mariotti C . The macular thickness and volume in glaucoma: an analysis in normal and glaucomatous eyes using OCT. Acta Ophthalmol Scand Suppl 2002; 236: 34–36.
Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS . Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci 2005; 46: 2012–2017.
Medeiros FA, Zangwill LM, Bowd C, Vessani RM, Susanna Jr R, Weinreb RN . Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol 2005; 139: 44–55.
Bagga H, Greenfield DS . Quantitative assessment of structural damage in eyes with localized visual field abnormalities. Am J Ophthalmol 2004; 137: 797–805.
Tan O, Li G, Lu AT, Varma R, Huang D . Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology 2008; 115: 949–956.
Acknowledgements
This work was supported by a grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (No. 309709/2007-5), Brasília, Brazil and from Coordenadoria de Aprimoramento do Pessoal de Nível Superior (CAPES), Brasília, Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Monteiro, M., Costa-Cunha, L., Cunha, L. et al. Correlation between macular and retinal nerve fibre layer Fourier-domain OCT measurements and visual field loss in chiasmal compression. Eye 24, 1382–1390 (2010). https://doi.org/10.1038/eye.2010.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.48
Keywords
This article is cited by
-
A comparison of macular ganglion cell and retinal nerve fibre layer optical coherence tomographic parameters as predictors of visual outcomes of surgery for pituitary tumours
Pituitary (2022)
-
Assessment of inner retina layers thickness values in eyes with pituitary tumours before visual field defects occur
Eye (2021)
-
Retinal layers in prolactinoma patients: a spectral-domain optical coherence tomography study
International Ophthalmology (2021)
-
Circumpapillary and macular vessel density assessment by optical coherence tomography angiography in eyes with temporal hemianopia from chiasmal compression. Correlation with retinal neural and visual field loss
Eye (2020)
-
Optical coherence tomography retinal ganglion cell complex analysis for the detection of early chiasmal compression
Pituitary (2018)