Abstract
Aims
To analyse the incidence of glaucoma in children undergoing cataract surgery and determine whether early surgery is associated with increased risk of glaucoma.
Methods
A retrospective chart review of all children aged 14 years or less who had surgery for congenital or developmental cataract at one unit over the last 20 years. The children were divided into three groups; group 1 consisting of children aged ⩽50 days at surgery, group 2 those aged 51 days to 1 year, and group 3 aged 1–14 years.
Results
We identified a total of 104 eyes of 74 children. The medical records for 100 eyes (71 children) were available for review. In all, 17 eyes (12 children) were aged ⩽50 days at surgery, none of which have developed glaucoma. Group 2 consisted of 28 eyes (17 children) with one patient developing glaucoma in both eyes 11 years after surgery. Group 3 consisted of 55 eyes (42 children), none of which have developed glaucoma. After a median follow-up period of 4.9 years (range 0.6–19.6 years, mean 6.4 ± 5.2 years) 2% of eyes had developed glaucoma. There was no significant difference in the length of follow-up between groups (H=2.979, P=0.22, Kruskal–Wallis Test).
Conclusions
There was a low incidence of glaucoma in our series and this was not increased in those having surgery in the first 6 weeks of life. Our findings contribute further evidence for the variability in prevalence of glaucoma after paediatric cataract extraction in the literature and suggest that factors other than age at surgery are important risk factors for this condition.
Similar content being viewed by others
Introduction
Glaucoma has been reported to occur in 6–50% of children after paediatric cataract extraction.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 It is not clear why glaucoma develops in these children; however, it appears to be a consequence of surgery rather than being directly related to the cataract itself. Children with untreated isolated congenital cataract do not tend to develop glaucoma16 and patients with bilateral congenital cataracts who only have unilateral surgery tend to develop glaucoma only in the operated eye.17 When glaucoma occurs the outcome is frequently poor and medical treatments are often insufficient.18
A majority of the studies show that infants undergoing cataract surgery during the first year of life have an increased risk of glaucoma compared with those that are older at the time of surgery.19, 20 Some have reported that surgery before 4 to 6 weeks of age confers a particularly high risk and have advocated that surgery be delayed until the second month of life, however, delaying surgery may deprive the child of the visual stimuli important for development and the avoidance of amblyopia.21
The purpose of this retrospective chart review was to analyse the incidence of glaucoma in children undergoing congenital and developmental cataract surgery at our institution and determine whether early surgery was associated with an increased risk of glaucoma.
Materials and methods
A retrospective chart review was conducted of children aged 14 years or less who had surgery for congenital or developmental cataract in one unit over the last 20 years. Patients were identified by a search of surgical log-books dating from 1 June 1987 to 1 January 2009 and from clinical coding records from 1 January 1998 to 1 January 2009. We also searched computerised clinic letter summaries available between 1 January 2003 and 1 January 2009. Patient case notes were obtained to allow the collection of data relevant to the study. Patients with a follow-up of <6 months after surgery were excluded. The children were divided into three groups according to age at surgery. Group 1 included those aged 50 days and under; group 2, those aged 51 days to 365 days and; group 3, those aged from 366 days to 14 years of age. The grouping of the children by age at time of surgery was set out in the study protocol with the aim that we would be able to compare glaucoma risk between groups. The age range for each group was decided based on the results of previous studies indicating that (a) surgery during the first year of life is associated with an increased risk of glaucoma19, 20 and (b) that surgery during the first 4 to 6 weeks may be associated with particularly high risk.21 We included both aphakic and pseudophakic children in the study, in addition to children with persistent hyperplastic primary vitreous (PHPV). We excluded patients with secondary cataracts, for example, because of trauma, or uveitis, and those eyes with pre-existing glaucoma. Patients who developed glaucoma or ocular hypertension after cataract extraction were identified.
Surgical technique
Surgery was performed by two surgeons (GW and SA). There was some variation in surgical technique depending on the age of the patient and the year of surgery. The youngest patients were treated with a pars ciliaris or limbal lensectomy and anterior vitrectomy, with care taken to remove as much lens matter as possible. The remaining patients were treated with lens aspiration with or without primary posterior capsulotomy and anterior vitrectomy. The standard postoperative regime consisted of 2 mg of subconjunctival betnesol, and prednisolone 1% eye drops four times per day for 1 month. Since March 2004, a more intensive steroid regime has been implemented for children <1 year of age consisting of 2 mg per 0.5 ml of intracameral dexamethasone at the end of the procedure and 1 mg/kg oral prednisolone for 5 days after surgery (used for 11 eyes included in this series).
Follow-up and glaucoma assessment
This was a retrospective case note review and therefore the follow-up schedule was variable. Children were observed the day after surgery and were closely observed in the immediate postoperative period. Long-term follow-up was approximately 6 monthly and consisted of orthoptic assessment, cycloplegic refraction, and fundoscopy, including optic disc assessment. Owing to the difficulties of measuring intraocular pressure (IOP) in young children, IOP measurements were not possible at every visit for every child. It was, however, normally possible to measure the IOP in infants using a lid speculum and Tono-Pen (Reichert. Inc, Seefeld, Germany) or Perkins tonometer (Haag-Streit UK Ltd, Harlow, Essex), while older children better tolerated Goldman tonometry. We performed examination under anaesthesia for children who were not cooperative enough to allow examination in the clinic, however, we did not anaesthetise children for the sole purpose of measuring IOP. If there were no features suggestive of glaucoma, children were observed until they were old enough to allow applanation tonometry.
Primary outcome measure
The primary outcome measure was the diagnosis of glaucoma, which was based on the clinician's decision to initiate treatment for raised IOP and glaucomatous optic disc changes.
Secondary outcome measure
The secondary outcome measure was ocular hypertension, which was defined as an IOP of >25 mm Hg, confirmed on re-testing. We chose a pressure of >25 mm Hg as our definition of ocular hypertension to allow comparison of our results with previous studies that had used the same definition.3, 10, 22
Results
Identification of patients
We identified a total of 104 eyes of 74 children who had had surgery for congenital or developmental cataract (Table 1). There were 38 males and 36 females. For the period 1 January 1998 to 1 January 2009, a total of 67 eyes were identified; 65 eyes from clinical coding records, 65 from the theatre log book, and 63 eyes from both sources. Capture–recapture analysis indicated that the likely total number of eyes undergoing surgery between 1 January 1998 and 1 January 2009 was 67 (95% CI 66.74–67.26). This suggests that our method for patient identification is reliable. An additional 37 eyes were identified from the clinic letters and from the theatre logbooks before 1 January 1998.
Case records were obtained for 96% of children (71/74 children and 100/104 eyes). The case records that we were unable to obtain were for three children who had had surgery in the 1980s whose notes had unfortunately been destroyed. Of the remaining children, 17 eyes (12 children) were aged ⩽50 days at surgery, 28 eyes (17 children) were aged 51–365 days, and 55 eyes (42 children) were aged 366 days to 14 years (Tables 1 and 2).
Surgical technique
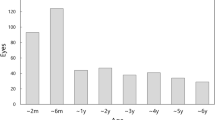
Forty-seven children had lensectomy at a median age of 67 days (range 6 days to 5.9 years, mean 146.9±34.8 days) (Figure 1). Children treated with lens aspiration, primary posterior capsulotomy, and anterior vitrectomy had a median age of 5.2 years at surgery (range 80 days to 13.8 years, mean 5.1±3.7 years). Children who had simple lens aspiration had a median age at surgery of 6 years (range 1.5–13.9 years, mean 7.5±4 years). An intraocular lens (IOLs) was inserted in 69.1% of eyes (38/55) in group 3 and 10.7% of eyes (3/28) in group 2. One child in group 1 had an attempted IOL insertion but this was abandoned because of instability of the capsule; the IOL was removed and capsulotomy and anterior vitrectomy completed. Two children in group 1 had poorly dilating pupils and required iris hook insertion. Seventeen children developed a complication or underwent a secondary surgical procedure (including secondary lens implantation) (Table 3). Nineteen children required a YAG laser capsulotomy.
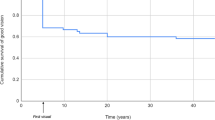
Follow-up
The median follow-up for all patients was 4.9 years (mean 6.4 ±5.1 years, range 0.6–19.6 years) (Figure 2). There was no significant difference in the length of follow-up between groups (P=0.16, H=3.665 Kruskal–Wallis Test). IOP was measured a mean number of 3.5 times per child during the follow-up period (range from 0 to 25). We were unable to measure the IOP in two children in group 1. During the course of this study, when it became apparent that these children had not had their IOP measured successfully, the families were contacted and invited for examination. Unfortunately, it was still not possible to obtain an accurate IOP measurement. We were, however, able to establish that these children had no clinical features suggestive of glaucoma with healthy optic disc appearances.
Incidence of glaucoma
At the time of most recent follow-up no patients in group 1 or group 3 had developed glaucoma. Glaucoma was diagnosed in both eyes of one patient (2/28, 7.1% of eyes) in group 2. Therefore, only 2 out of 100 eyes (2%) have developed glaucoma over a median follow-up period of 4.9 years.
The patient in this series with glaucomatous optic neuropathy had cerebral palsy, epilepsy, and bilateral cataract. She was 156 days old at the time of left pars ciliaris lensectomy in April 1989 and had a right lensectomy 6 days later. Although the initial surgical objective had been to remove as much lens matter as possible, she required further surgery to remove of a pupillary membrane in the left eye 3 months later. In spite of regular outpatient visits and examination under anaesthesia, glaucoma was not diagnosed until 2001, 11.6 years after surgery, when the child presented acutely with a painful, red left eye. On examination she was found to have a small hyphaema and IOP of 35 mm Hg. Examination under anaesthesia revealed iris rubeosis and synechiael angle closure. Ten months later glaucoma was also diagnosed in the right eye after the IOP rose to 38 mm Hg. The left eye responded poorly to medical and surgical treatment and is now phthisical. The right eye has fared better and 19.6 years after surgery has a well-controlled IOP with medical treatment alone and visual acuity of 0.4 Log Mar with spectacles.
One patient in group 1 developed ocular hypertension. This child had a right lensectomy at 35 days of age and after 2.85 years of follow-up was noted to have an IOP of 26 mm Hg. Thus far this child has showed no other features of glaucoma and is being monitored without treatment. There were no patients in group 2 or group 3 with ocular hypertension.
Discussion
Previous series have shown a large variation in the percentage of children who develop glaucoma after surgery for congenital or developmental cataract (Table 4). In this series, the incidence of glaucoma is lower than in previous reports. After a median follow-up period of 5 years, was only 2% of eyes have developed glaucoma and as yet none of the children operated on during the first 50 days of life have developed glaucoma.
The reasons for the low incidence of glaucoma in this series are not clear. There are many factors that may influence the reported risk of glaucoma after paediatric cataract surgery. Some of the variation between studies could relate to differences in the length of follow-up. Glaucoma may develop many years after surgery so we would expect studies with longer follow-up to report higher incidences of glaucoma, however, this is not always the case.6 For example, Chen et al19 found 58.7% of children had raised IOP after lensectomy after a mean follow-up period of 10 years. In contrast, Rabiah et al12 who had similar length of follow-up found only 20.7% of children developed raised IOP. Although the follow-up period in our series is shorter than that achieved by Chen et al and Rabiah et al, it is comparable to most other studies. Furthermore, we had the longest follow-up in the youngest age group with the median length of follow-up for group 1 patients at over 7 years. The British Congenital Cataract Study found that the median time to development of postoperative glaucoma was only 1.34 years (range 0.39 months to 6.73 years)23 therefore, we would expect our follow-up period to be long enough to detect most cases of glaucoma. It is important to point out that the child in our series, who developed glaucoma, did not do so until over 11 years postoperatively. Given that the mean follow-up period for our patients was just 5 years, it is certainly possible that the prevalence of glaucoma in our group may increase with time. Therefore, in spite of the early median time to glaucoma found in the British Congenital Cataract Study, lifelong follow-up for children who have had congenital cataract extraction remains important.
Comparison between studies is further complicated by differences in glaucoma diagnostic criteria. Some studies have examined raised IOP while others define glaucoma by decision to treat or by raised IOP and associated findings such as corneal oedema or glaucomatous optic disc changes.22 We defined glaucoma by decision to treat but also recorded any IOP measurement of >25 mm Hg. We found one patient with ocular hypertension and it is possible that this child may go on to develop glaucoma. Egbert et al6 examined the conversion rates from ocular hypertension to glaucoma and found that the rate of progression from ocular hypertension to glaucoma over 7 years was 23%.
Variation in the reported incidence of glaucoma may also be due to differences in study populations. For example, certain cataract morphologies and co-existent ocular abnormalities may be associated with an increased glaucoma risk.24, 25 We were not able to examine the role of cataract morphology or microcornea as it proved difficult to ascertain this information retrospectively. This is a shortcoming of our study. We decided to include patients with PHPV as previous investigators had found PHPV not to be associated with an increased risk of glaucoma.21 As the number of eyes developing glaucoma in this series was low, there was insufficient power to assess the effect of these conditions on glaucoma risk.
This study was also limited by the retrospective design, lack of standard follow-up protocol, and the loss of patients to follow-up. It is possible that the two children who have yet to have IOP measurements have ocular hypertension and that some of the IOP measurements we were able to obtain may be inaccurate. Where possible we assessed intraocular pressure using the Goldman tonometer, however, in younger children the Perkins tonometer and Tono-Pen were used. Further bias may have been introduced by the use of more than one type of tonometer, particularly the Tono-Pen, which has been shown to overestimate higher IOPs by a mean of almost 6 mm Hg in one study of children with congenital glaucoma.26 IOP measurements may also be influenced by attempted eyelid closure27 and in some children may not possible at all without resorting to examination under anaesthesia. Ideally, we would have performed pachymetry for all of our children. A previous study has shown that aphakic and pseudophakic patients have significantly thicker corneas than age-matched controls.28 This may lead to some children being labelled ocular hypertensive who in fact have a normal IOP.
The child who developed glaucoma in our series did not do well. Previous series of children with aphakic glaucoma concur that when glaucoma occurs the long-term outcome is poor. Bhola et al18 found one-quarter of eyes with aphakic glaucoma required pressure lowering surgery. In all, 60% of eyes that require surgery need more than one procedure.
There are many reports in the literature suggesting that children who undergo lensectomy during the first year of life have an increased risk of glaucoma compared with those that are older at the time of surgery.19, 20 For example, Rabiah12 found a decreasing risk of glaucoma with increasing patient age. Over a mean follow-up of 9 years, glaucoma developed in 21% (118/570) of eyes including 37% if surgery was performed at <9 months of age, 14% with surgery between 9 months and 2 years of age, and only 9% with surgery between 2 and 3 years of age.12 Robb, Mills, and Walton8, 9, 20 have all reported similar findings. Swamy et al10 conducted a 20-year retrospective review of children undergoing lensectomy for congenital cataract. Children aged 9 months or less at surgery had a 10-year prevalence of glaucoma of 29.9% compared with 5% for those over 9 months of age. Lately, there has been concern that surgery during the first month of life confers a particularly high risk of aphakic glaucoma.7, 14, 22, 29 Vishwanath et al,21 found that lensectomy for bilateral infantile cataract during the first month was associated with an increased risk of aphakic glaucoma compared with delayed surgery. The 5-year risk of glaucoma in at least one eye after bilateral lensectomy was 50% with surgery in the first month of life but when surgery was delayed to later in the first year the risk of glaucoma was only 14.9%. Following such observations, it has been suggested that a delay in surgery in young infants with bilateral cataract may be prudent to reduce the risk of glaucoma.21 However, this concept is complicated by the large variation between studies. For example, one group has even found glaucoma to be less prevalent in children undergoing surgery in the first 2 weeks of life compared with those undergoing surgery at 2–12 weeks.30 The lack of consensus and different recommendations as to the optimal time for surgery suggests that the risk of glaucoma is determined by factors other than the age of surgery.
Walton et al20 examined 65 children with aphakic glaucoma and found glaucoma was frequently associated with residual lens tissue. The child who developed glaucoma in our series had a complicated surgical course and required surgical removal of a papillary membrane 3 months after her initial lensectomy. It is likely that this child had significant residual lens tissue but whether this contributed to glaucoma is not clear especially as glaucoma did not develop until over 10 years later. Previous studies have indicated a bimodal onset of glaucoma after congenital cataract surgery with early-onset glaucoma associated with angle closure presenting during the first year of life and open angle late-onset glaucoma presenting years later.15, 31 Ken Nischal's group have recently shown that when congenital cataract surgery is traumatic the risk of glaucoma may increase.33 In a series of 98 eyes of 62 children who had congenital cataract surgery during the first year of life, 9.8% of aphakic eyes and 13.5% of pseudophakic eyes developed glaucoma over a mean follow-up period of 2.5 years. Although there was no statistically significant effect of age on the rate of early-onset glaucoma there were four cases in which an IOLs was placed in the bag but had to be removed and all four of these eyes developed glaucoma within 14 weeks.
Intraocular inflammation is a well-recognised cause of raised IOP and may lead to permanent structural changes in the angle and at the pupillary margin. A possible explanation for the low incidence of glaucoma in our series is that the surgical objective in the younger patients was complete removal of all lens matter without regard to the potential difficulty that this might create in placing a secondary intraocular lens. We would expect this approach to minimise residual lens tissue and postoperative inflammation. Perioperative steroids are also important to minimise intraocular inflammation. Intensive topical steroids have been the mainstay, however, some groups have advocated adjuvant systemic, periocular, and intracameral steroids.34 Although we had a low incidence of glaucoma in children undergoing surgery during the 1980s and 1990s, we changed to a more intensive steroid regimen for the younger children. We hoped this would further minimise inflammation and hopefully further reduce the risk of glaucoma. Parents often struggle when required to frequently instil eye drops and missed application is less likely to be a problem with systemically administered medication. This may also provide parents of aphakic children with more time to master the demands of the contact lens routine.
Conclusion
Glaucoma is a serious complication of paediatric cataract surgery, which can develop at any time postoperatively. Previous studies have suggested that performing surgery early in life is a risk factor for the development of aphakic glaucoma. In our series, we found a low incidence of glaucoma, even in those children undergoing cataract surgery at a very young age. The results of this study should be interpreted with some caution because of the limitations of the study design and the relatively small number of children in the youngest age group, however, it would seem that factors other than age at cataract surgery are important in determining whether a child will develop glaucoma or not.

References
Egbert JE, Wright MM, Dahlhauser KF, Keithahn MA, Letson RD, Summers CG . A prospective study of ocular hypertension and glaucoma after pediatric cataract surgery. Ophthalmology 1995; 102 (7): 1098–1101.
Chrousos GA, Parks MM, O’Neill JF . Incidence of chronic glaucoma, retinal detachment and secondary membrane surgery in pediatric aphakic patients. Ophthalmology 1984; 91 (10): 1238–1241.
Chen TC, Walton DS, Bhatia LS . Aphakic glaucoma after congenital cataract surgery. Arch Ophthalmol 2004; 122 (12): 1819–1825.
Magnusson G, Abrahamsson M, Sjöstrand J . Glaucoma following cataract surgery (an 18-year longitudinal follow-up). Acta Opthalmol Scand 2000; 78: 65–70.
Chen TC, Bhatia LS, Halpern EF, Walton DS . Risk factors for the development of aphakic glaucoma after congenital cataract surgery. Trans Am Ophthalmol Soc 2006; 104: 241–251.
Egbert JE, Christiansen SP, Wright MM, Young TL, Summers CG . The natural history of glaucoma and ocular hypertension after pediatric cataract surgery. J AAPOS 2006; 10 (1): 54–57.
Keech RV, Tongue AC, Scott WE . Complications after surgery for congenital and infantile cataracts. Am J Ophthalmol 1989; 108: 136–141.
Mills MD, Robb RM . Glaucoma following childhood cataract surgery. J Pediatr Ophthalmol Strabismus 1994; 31 (6): 355–360; discussion 361.
Robb RP, Petersen RA . Outcome of treatment for bilateral congenital cataracts. Ophthalmic Surg 1992; 23: 650–656.
Swamy BN, Billson F, Martin F, Donaldson C, Hing S, Jamieson R et al Secondary glaucoma after paediatric cataract surgery. Br J Ophthalmol 2007; 91 (12): 1627–1630.
Simon JW, Mehta N, Simmons ST, Catalano RA, Lininger LL . Glaucoma after pediatric lensectomy/vitrectomy. Ophthalmology 1991; 98 (5): 670–674.
Rabiah PK . Frequency and predictors of glaucoma after pediatric cataract surgery. Am J Ophthalmol 2004; 137 (1): 30–37.
Johnson CK, Petersen RA . Prevalence of glaucoma after surgery for PHPV and infantile cataracts. J Pediatr Ophthalmol Strabismus 1996; 33: 14–17.
Lundvall AK, Kugelberg U . Outcome after treatment of congenital bilateral cataract. Acta Ophthalmol Scand 2002; 80: 593–597.
Asrani SG, Wilensky JT . Glaucoma after congenital cataract surgery. Ophthalmology 1995; 102 (6): 863–867.
Mori M, Keech RV, Scott WE . Glaucoma and ocular hypertension in pediatric patients with cataracts. J AAPOS 1997; 1 (2): 98–101.
Simon J . Discussion (glaucoma following childhood cataract surgery). J Pediatr Ophthalmol Strabismus 1994; 31: 361.
Bhola R, Keech RV, Olson RJ, Petersen DB . Long-term outcome of pediatric aphakic glaucoma. J AAPOS 2006; 10 (3): 243–248.
Chen TC, Bhatia LS, Halpern EF, Walton DS . Risk factors for the development of aphakic glaucoma after congenital cataract surgery. J Pediatr Ophthalmol Strabismus 2006; 43 (5): 274–280; quiz 306–277.
Walton DS . Pediatric aphakic glaucoma: a study of 65 patients. Trans Am Ophthalmol Soc 1995; 93: 403–413; discussion 413–420.
Vishwanath M, Cheong-Leen R, Taylor D, Russell-Eggitt I, Rahi J . Is early surgery for congenital cataract a risk factor for glaucoma? Br J Ophthalmol 2004; 88 (7): 905–910.
Magnusson G, Abrahamsson M, Sjostrand J . Glaucoma following congenital cataract surgery: an 18-year longitudinal follow-up. Acta Ophthalmol Scand 2000; 78 (1): 65–70.
Chak M, Rahi JS . Incidence of and factors associated with glaucoma after surgery for congenital cataract: findings from the British Congenital Cataract Study. Ophthalmology 2008; 115 (6): 1013–1018 e1012.
Parks MJ, Johnson DA, Reed GW . Long-term visual results and complications in children with aphakia. A function of cataract type. Ophthalmology 1993; 100: 826–840.
Phelps CD, Arafat NI . Open-angle glaucoma following surgery for congenital cataracts. Arch Ophthalmol 1977; 95 (11): 1985–1987.
Levy J, Lifshitz T, Rosen S, Tessler Z, Biedner BZ . Is the Tono-Pen accurate for measuring intraocular pressure in young children with congenital glaucoma? J AAPOS 2005; 9 (4): 321–325.
Jamal KN, Gurses-Ozden R, Liebmann JM, Ritch R . Attempted eyelid closure affects intraocular pressure measurement in open-angle glaucoma patients. Am J Ophthalmol 2002; 134 (2): 186–189.
Simsek T, Mutluay AH, Elgin U, Gursel R, Batman A . Glaucoma and increased central corneal thickness in aphakic and pseudophakic patients after congenital cataract surgery. Br J Ophthalmol 2006; 90 (9): 1103–1106.
Michaelides M, Bunce C, Adams GG . Glaucoma following congenital cataract surgery--the role of early surgery and posterior capsulotomy. BMC Ophthalmol 2007; 7: 13.
Watts P, Abdolell M, Levin AV . Complications in infants undergoing surgery for congenital cataract in the first 12 weeks of life: is early surgery better? J AAPOS 2003; 7 (2): 81–85.
Kuhli-Hattenbach C, Luchtenberg M, Kohnen T, Hattenbach LO . Risk factors for complications after congenital cataract surgery without intraocular lens implantation in the first 18 months of life. Am J Ophthalmol 2008; 146 (1): 1–7.
Khan AO, Al-Dahmesh S . Age at the time of cataract surgery and relative risk for aphakic glaucoma in nontraumatic infantile cataract. J AAPOS 2009; 13 (2): 166–169.
Wong IB, Sukthankar VD, Cortina-Borja M, Nischal KK . Incidence of early-onset glaucoma after infant cataract extraction with and without intraocular lens implantation. Br J Ophthalmol 2009; 93 (9): 1200–1203.
Lee SY, Chee SP, Balakrishnan V, Farzavandi S, Tan DT . Surodex in paediatric cataract surgery. Br J Ophthalmol 2003; 87 (11): 1424–1426.
Acknowledgements
Neither the corresponding author nor any co-author has a financial interest in the subject matter. This work was presented in part at the World Congress of Paediatric Ophthalmology, Barcelona, Spain, September 2009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tatham, A., Odedra, N., Tayebjee, S. et al. The incidence of glaucoma following paediatric cataract surgery: a 20-year retrospective study. Eye 24, 1366–1375 (2010). https://doi.org/10.1038/eye.2010.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.46
Keywords
This article is cited by
-
Outcomes of the infantile cataract surgery: case series with a 5-year follow-up
International Ophthalmology (2022)
-
The impact of late-treated pediatric cataract on intraocular pressure
International Ophthalmology (2021)
-
Cataract management in children: a review of the literature and current practice across five large UK centres
Eye (2020)
-
Prospective analysis of the predictors of glaucoma following surgery for congenital and infantile cataract
Eye (2019)
-
Prognostic factors of pediatric glaucoma: a retrospective study
International Ophthalmology (2019)