Abstract
Purpose
To investigate the development of polypoidal lesions using indocyanine green angiography (IA) in eyes with typical age-related macular degeneration (AMD).
Methods
We retrospectively reviewed the medical records of 47 consecutive patients (47 eyes) with typical AMD who had been followed up with IA for at least 2 years.
Results
At the initial visit, although all eyes showed classic and/or occult choroidal neovascularization (CNV) associated with AMD, no eyes showed polypoidal lesions by IA. However, during follow-up, 13 (27.7%) of the 47 eyes did show polypoidal lesions. All polypoidal lesions developed at the edge of persistent CNV or, more often, at the terminus of recently progressed CNV. Of 12 eyes with a final lesion area >8 disc area, 7 (58.3%) showed newly developed polypoidal lesions. In the eyes with these newly developed polypoidal lesions, the mean area of the vascular lesion had extended significantly from 10.50±7.88 mm2 to 20.87±10.21 mm2 during follow-up (P=0.0018).
Conclusion
The current observation suggests that IA of active AMD sometimes reveals polypoidal lesions if there is progression of the CNV in the subretinal pigment epithelium space.
Similar content being viewed by others
Introduction
Initially, polypoidal choroidal vasculopathy (PCV) was described as a new clinical entity with a unique form of choroidal vascular abnormality.1, 2, 3 As Uyama et al4, 5 proposed that PCV is a variant of choroidal neovascularization (CNV) in exudative age-related macular degeneration (AMD), it has been generally believed that the vascular components in PCV are a type of CNV, although the pathogenesis of PCV is not fully understood.6, 7 In general, PCV is diagnosed when polypoidal lesions are seen by indocyanine green angiography (IA) at the terminus of typical branching vascular networks.8 IA is essential for the study of the vascular lesion of PCV because most of the vascular components of PCV are located beneath the retinal pigment epithelium;8 hence, with fundus examination or fluorescein angiography (FA), it is often difficult to distinguish macular PCV from exudative AMD.9, 10, 11
An increasing number of reports has recently shown that anti-vascular endothelial growth factor (VEGF) therapy can reduce the exudative change and lesion size in exudative AMD, and can result in visual recovery.12, 13, 14, 15, 16, 17, 18 However, some eyes with exudative AMD are refractory to repeated anti-VEGF therapy.19, 20 Clinicians sometimes see cases of typical AMD with a large lesion that shows a poor response to treatment and that shows progression, which results in a poor visual prognosis. When we performed IA on such AMD cases with large lesions, we sometimes noted knob-like hyperfluorescent lesions at the end of progressing CNV, which resembled the polypoid lesions typically seen in PCV.21 Cho et al19 reported that IA revealed these polypoidal lesions in 12 cases of exudative AMD with a poor response to anti-VEGF therapy.
In the clinical setting, FA is essential to make a diagnosis and to evaluate lesion size and its activity in exudative AMD.22 Even in exudative AMD, IA often provides useful information about vascular lesions, especially those located beneath the retinal pigment epithelium.23, 24 So far, however, limited information is available using IA on long-term observations of the vascular components in typical AMD. In this study, we studied the vascular lesions of typical AMD using IA, to elucidate the rate and characteristics of eyes that were initially diagnosed as having typical AMD and that subsequently developed polypoidal lesions.
Patients and methods
For this observational case study, we retrospectively reviewed the medical records of 47 consecutive patients (47 eyes) with typical AMD who initially visited the Macula Service, Department of Ophthalmology, Kyoto University Hospital between October 2004 and October 2007 and whose eyes had been examined with both FA and IA for more than 2 years after the initial visit. During this period, 116 patients with typical AMD initially visited our clinic. In all, 69 patients were excluded from the current study because of a short follow-up period or because they lacked FA or IA during the more than 2 years since the initial visit. When both eyes showed typical AMD, only one eye was selected randomly for inclusion in the current study. This study was approved by the Institutional Review Board at the Kyoto University Graduate School of Medicine and adhered to the tenets of the Declaration of Helsinki.
The diagnosis of typical AMD was made based on fundus photographs, FA, IA, and optical coherence tomography (OCT), which showed type 1 and/or type 2 CNV. Eyes with other macular abnormalities (such as PCV, retinal angiomatous proliferation, pathological myopia, idiopathic CNV, presumed ocular histoplasmosis, angioid streaks, and other secondary CNV) were excluded from the current study. When reddish-orange nodules were seen by ophthalmoscopic examination or polypoidal lesions were seen by IA, the eye was excluded from the current study. Eyes that had been previously treated with focal laser photocoagulation, photodynamic therapy, vitrectomy, radiation therapy, or anti-VEGF therapy were also excluded from this study.
At the initial visit, all patients had undergone a comprehensive ophthalmological examination, including measurement of best-corrected visual acuity (VA), determination of intraocular pressure, indirect ophthalmoscopy, slitlamp biomicroscopy with a contact lens, and OCT. After fundus photographs were taken, FA and IA were performed on each patient using a confocal laser scanning system (HRA-2, Heidelberg Engineering, Dossenheim, Germany). VA measurement and OCT examination were performed on each patient at each follow-up visit; FA and IA were performed if necessary, and all patients in the current study were examined with both FA and IA several times during their follow-up.
In this study, the greatest linear dimension (GLD) and area of the lesion were determined based on the IA using software that was built into the HRA-2. GLD was defined as covering the entire CNV lesion, including type 1 and type 2 CNV, polypoidal lesions, and the branching vascular network. The area of the CNV lesion was measured manually using software in HRA-2. Pigment epithelial detachment, without underlying CNV, was not included in the measurement of GLD or in the area of the lesion. In the current study, one optic disc area (DA) is considered to be 2.54 mm2 on the basis of one optic disc diameter being 1.8 mm.
Using OCT images, we performed two measurements using a caliper that was built into the software of the OCT machine; using these calipers, foveal thickness and thickness of the neurosensory retina in the fovea were measured. Foveal thickness was defined as the distance between the vitreoretinal interface and the retinal pigment epithelium; thickness of the neurosensory retina was defined as the distance between the vitreoretinal interface and the tip of the outer portion of the inner and outer segments of the photoreceptors.
In the current study, measurement values obtained at the initial visit were compared with those obtained at the final examination. To evaluate progression of the vascular lesions, the initial area of the CNV lesion and that of the GLD were compared with final values. To evaluate activity of the disease, we also compared the initial OCT measurement (foveal thickness and thickness of the neurosensory retina) with values obtained at the final visit. To compare the difference in VA, vision as measured with a Landolt chart was converted to a logarithm of the minimal angle of resolution (logMAR).
Statistical analysis was performed using software designed for this purpose (StatView, version 5.0; SAS Institute, Cary, NC, USA). A P-value <0.05 was considered to be statistically significant.
Results
In the current study, 47 eyes of 47 patients (36 men and 11 women) with typical AMD were examined; the patients ranged in age from 56 to 90 years (72.8±7.5 years). The follow-up period ranged from 24 to 59 months (43.9±10.3 months). All patients were examined with FA and IA repeatedly during their follow-up, ranging from 2 to 10 times (4.6±1.8 times). Table 1 shows characteristics of patients who were eligible for this study. Mean baseline VA (logMAR) was 0.70±0.53, and the mean initial areas of the lesion and GLD were 8.68±7.75 mm2 and 3850±1627 μm, respectively. Figure 1 shows the relationship of area of the lesion and of the GLD obtained at the initial visit and at final examination. The initial area of the lesion (R=0.7347, P<0.0001) and initial GLD (R=0.7349, P<0.0001) were correlated closely with final values.
(a) Correlation between initial and final areas of the lesion in eyes with typical age-related macular degeneration. Initial areas are significantly correlated with final areas of the lesion (R=0.7347, P<0.0001). (b) Correlation between initial and final greatest linear dimension (GLD) in eyes with typical age-related macular degeneration. Initial GLD is significantly correlated with final GLD (R=0.7349, P<0.0001). One disc area (DA) is estimated to be 2.54 mm2 on the basis of one optic disc diameter being 1.8 mm. Open diamonds represent eyes without polypoidal lesions, and closed diamonds represent eyes with polypoidal lesions.
At the initial visit, although all eyes of the patients involved showed classic and/or occult CNV associated with AMD, no eyes showed polypoidal lesions by IA. During the follow-up period, 34 eyes were initially treated with photodynamic therapy, and 5 were initially treated with anti-VEGF therapy. In spite of these treatments, however, some eyes with typical AMD showed extension of the vascular components with an exudative change. The mean area of the neovascularization increased from 8.68±7.75 mm2 to 14.28±10.57 mm2 during follow-up (P<0.0001). Moreover, during the follow-up, 13 (27.7%) of the 47 eyes showed polypoidal lesions by IA. All of these polypoidal lesions developed either at the edge of persistent CNV (Figure 2) or, more often, at the terminus of the newly progressed CNV (Figures 3 and 4). Of 12 eyes with a final CNV area >8 DA, 7 (58.3%) showed newly developed polypoidal lesions (Figure 1).
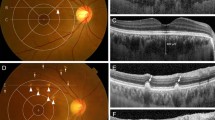
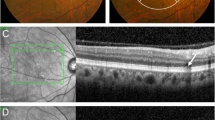
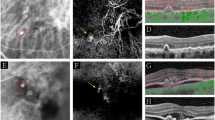
Development of polypoidal lesions at the border of persistent choroidal neovascularization (CNV) in a right eye with typical age-related macular degeneration. (a) Fundus photograph at the initial visit shows a multi-lobed serous pigment epithelial detachment (PED). Visual acuity was 0.8 in this eye. (b) Fluorescein angiography (FA) at the initial visit shows only hyperfluorescent areas associated with the PED. (c) Indocyanine green angiography (IA) shows CNV (arrow) at the edge of the PED; no polypoidal lesions are seen. (d) Fundus photograph at 34 months after the initial visit. With two photodynamic treatments, activity of the CNV has regressed. Visual acuity was now 0.2 in the right eye. (e) At 50 months after the initial visit, new serosanguineous PEDs (arrows) have developed at the upper side and at the inferotemporal side of the subfoveal disciform scar. (f) IA shows multiple polypoidal lesions (arrows) at the edge of the persistent CNV. Visual acuity was 0.1 in this eye.
Development of polypoidal lesions at the terminus of newly progressed choroidal neovascularization (CNV) in a left eye with typical age-related macular degeneration. (a) Fundus photograph at the initial visit; at which time visual acuity in this eye was 1.2. (b) Fluorescein angiography (FA) at the initial visit shows inactive occult CNV. (c) Indocyanine green angiography (IA) shows no polypoidal lesions. (d) At 34 months after the initial visit, visual acuity had decreased to 0.15. Fundus photograph shows an active exudative change at the temporal side of a subfoveal disciform scar. (e) FA shows large subfoveal classic CNV with temporal active leakage from the CNV (arrow). (f) IA shows mature subfoveal CNV (long arrow). The CNV has progressed temporally and now forms multiple terminal bulbs, which are seen as polypoidal lesions (arrow). Three intravitreal injections of bevacizumab caused activity of the CNV to regress. (g) At 64 months after the initial visit, a new active lesion has developed on the upper border of the subfoveal disciform scar. Visual acuity, however, remained at 0.15. (h) FA shows newly developed CNV (arrow). (i) IA shows what are presumed to be newly developed polypoidal lesions (arrow).
Development of polypoidal lesions at the terminus of recently progressive choroidal neovascularization (CNV) in a right eye with typical age-related macular degeneration. (a) Fundus photograph at the initial visit shows atrophy of the retinal pigment epithelium. Visual acuity was 0.4 in the right eye. (b) Fluorescein angiography (FA) at the initial visit shows subfoveal occult CNV. (c) Indocyanine green angiography (IA) shows CNV beneath the fovea, but no polypoidal lesions are seen. With photodynamic therapy, activity of the CNV regressed. (d) At 24 months after the initial visit, a new active lesion has developed at the temporal side of the regressed CNV. Visual acuity at this time was 0.4 in the right eye. (e) IA reveals multiple polypoidal lesions (arrows) at the edge of the progressive CNV. (f) Magnified IA shows that some of the polypoidal lesions consist of coiled vessels (arrow).
Depending on the development of polypoidal lesions, we divided our patients with typical AMD into two groups—those with polypoidal lesions (n=13) and those without polypoidal lesions (n=34). Table 1 shows baseline and final measurement values of each group. There were no significant differences in gender or age between these two groups (P=0.9739 and P=0.8913). Furthermore, there were no differences in VA (P=0.6234), area of lesion (P=0.3262), or GLD (P=0.3269) at the initial visit. The mean area of the lesion in eyes with polypoidal lesions enlarged significantly from 10.50±7.88 mm2 to 20.87±10.21 mm2 during the follow-up (P=0.0018). The mean GLD in eyes with polypoidal lesions also progressed significantly from 4231±1692 μm to 5880±1947 μm (P=0.0032). Although mean areas of the lesion and GLD were increased in eyes without polypoidal lesions, the increases were significantly greater in eyes that developed polypoidal lesions (P=0.0036 and P=0.0035). In eyes with polypoidal lesions, however, final VA was not statistically different compared with that in eyes without polypoidal lesions (P=0.2303).
Discussion
PCV was first described as a new clinical entity with an associated unique form of choroidal vascular abnormality.1, 2, 3 Subsequently, however, based on histological specimens and OCT examinations, PCV is considered to be a unique form of CNV.25, 26 In general, presumed exudative AMD is diagnosed as PCV when IA shows polypoidal lesions at the terminus of the branching vascular network.8 In PCV, IA is essential to make the diagnosis and to precisely evaluate the entire vascular lesion.8 In the current study, polypoidal lesions developed in 27.7% of eyes with typical AMD—eyes that had shown no polypoidal lesions at the initial visit. All polypoidal lesions developed at the edge of persistent CNV or, more often, at the terminus of recently progressed CNV. The current observation suggests that active AMD may show polypoidal lesions, if there is progression in the subretinal pigment epithelium space.
The tip of the extending CNV, which is the most active lesion, typically shows vigorous leakage on FA.22 Even if the CNV is located beneath the retinal pigment epithelium, it sometimes shows a ‘classic’ appearance, as proposed in the updated clinicopathological classification of subretinal neovascularization by Gass.27 As molecules of indocyanine green readily attach to serum albumin,28 vigorous leakage is not seen usually by IA, even from active CNV,23, 24, 29 but CNV that is stained by indocyanine green may well appear as knobbed hyperfluorescent spots on IA.30 In our patients, the tips of progressive CNV associated with AMD that showed focal hyperfluorescence on IA might appear to be polypoidal lesions.
Another interpretation is also possible. We can assume that the branching vascular network and polypoidal lesions represent a variant of type 1 CNV associated with exudative AMD.4, 5 In the clinical setting, when no polypoidal lesions are seen, it is difficult to distinguish the branching vascular network from the type 1 CNV that may be associated with AMD.31 Clinically, some polypoidal lesions do regress spontaneously or, more commonly, after photodynamic therapy, but they tend to recur at the same location or at other terminals of the branching vascular network.6, 9, 32 In the current study, a diagnosis of typical AMD was made in all eyes, based on the initial IA. It is possible that some of our patients actually had PCV which was misdiagnosed, and that some showed polypoidal lesions during the follow-up. Recently, Maruko et al33 reported that among 349 patients with neovascular AMD, 20 (5.7%) had 1 eye with PCV, whereas the fellow eye showed typical AMD, but that PCV developed during the follow-up in 10 eyes that had typical AMD at the initial examination. As their patients had PCV lesions in the fellow eye, it is reasonable to assume that these eyes originally had PCV but did not meet the diagnostic criteria of PCV.33
Our patients had been examined with both FA and IA for more than 2 years after the initial visit. In the current study, the rate of those eyes that were refractory to treatment or that showed a recurrence of CNV after the successful initial treatment might be relatively high. Recently, Cho et al19 reported that polypoidal lesions were detected in 12 cases of exudative AMD with a poor response to anti-VEGF therapy. In their report, treatment with photodynamic therapy, photodynamic therapy/anti-VEGF combination therapy, or continued anti-VEGF monotherapy resulted in complete resolution of exudation in 9 of 12 eyes. In the current study, we showed that typical AMD refractory to treatment sometimes developed polypoidal lesions at the terminus of the newly progressed CNV. However, visual prognosis of these eyes was not poor. We treated eyes with polypoidal lesions primarily by photodynamic therapy or photodynamic therapy combined with anti-VEGF therapy. Today, anti-VEGF therapy is a primary treatment for typical AMD. In eyes with exudative AMD refractory to treatment, however, we suggest that clinicians should reconsider the treatment strategy after re-evaluation of the CNV by IA.
It is still controversial as to whether PCV is a distinct clinical entity or is simply a unique form of exudative AMD.6 In Asian populations, a number of reports have shown that genetic variations in ARMS2 and CFH are strongly associated with both AMD and PCV.34, 35, 36, 37, 38 In addition, Lima et al39 recently reported that the PCV phenotype in Caucasian patients is associated with the three major AMD-associated loci, including CFH, ARMS2, and CFB/C2 genes, and they suggested that PCV and AMD are genetically similar in these tested loci.39 Although Kondo et al40 have implicated the elastin gene as a susceptibility gene for PCV, it is generally believed that PCV and AMD share common genetic backgrounds, even though complications, treatment effect, and visual prognosis are somewhat different.6, 11 Indeed, PCV is a distinct clinical entity,9 although some advanced AMD cases do show features characteristic of PCV.33
Limitations of the current study are its retrospective nature and the various treatment regimens used. In the current study, active AMD sometimes showed polypoidal lesions at the terminus of the vascular lesion, when the neovascularization showed progression in the subretinal pigment epithelium space. In the clinical setting, eyes with a small PCV lesion often maintain good VA for a protracted period of time.41, 42 In contrast, cases of PCV with a large lesion that has a poor response to treatment and that shows progression, tend to have a poor visual prognosis.21 Our findings suggest that advanced AMD overlaps with PCV—at least with PCV that has a large vascular lesion.

References
Kleiner RC, Brucker AJ, Johnston RL . The posterior uveal bleeding syndrome. Retina 1990; 10: 9–17.
Stern RM, Zakov ZN, Zegarra H, Gutman FA . Multiple recurrent serosanguineous retinal pigment epithelial detachments in black women. Am J Ophthalmol 1985; 100: 560–569.
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B . Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990; 10: 1–8.
Uyama M, Matsubara T, Fukushima I, Matsunaga H, Iwashita K, Nagai Y et al. Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol 1999; 117: 1035–1042.
Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 2002; 133: 639–648.
Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA . Polypoidal choroidal vasculopathy. Surv Ophthalmol 2004; 49: 25–37.
Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 2003; 121: 1392–1396.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA . Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995; 15: 100–110.
Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA . The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 1997; 115: 478–485.
Yannuzzi LA, Wong DW, Sforzolini BS, Goldbaum M, Tang KC, Spaide RF et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol 1999; 117: 1503–1510.
Moorthy RS, Lyon AT, Rabb MF, Spaide RF, Yannuzzi LA, Jampol LM . Idiopathic polypoidal choroidal vasculopathy of the macula. Ophthalmology 1998; 105: 1380–1385.
Lee SY, Kim JG, Joe SG, Chung H, Yoon YH . The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol 2008; 22: 92–99.
Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, Kamei M et al. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 2008; 92: 70–73.
Lai TY, Chan WM, Liu DT, Luk FO, Lam DS . Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol 2008; 92: 661–666.
Tsujikawa A, Ooto S, Yamashiro K, Tamura H, Otani A, Yoshimura N . Treatment of polypoidal choroidal vasculopathy by intravitreal injection of bevacizumab. Jpn J Ophthalmol 2010; 54: 310–319.
Kokame GT, Yeung L, Lai JC . Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol 2010; 94: 297–301.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1432–1444.
Cho M, Barbazetto IA, Freund KB . Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol 2009; 148: 70–78.
Stangos AN, Gandhi JS, Nair-Sahni J, Heimann H, Pournaras CJ, Harding SP . Polypoidal choroidal vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. Am J Ophthalmol 2010; 150: 666–673.
Tateiwa H, Kuroiwa S, Gaun S, Arai J, Yoshimura N . Polypoidal choroidal vasculopathy with large vascular network. Graefes Arch Clin Exp Ophthalmol 2002; 240: 354–361.
Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for Evaluation and Treatment in the Macular Photocoagulation Study. Arch Ophthalmol 1991; 109: 1242–1257.
Yannuzzi LA, Slakter JS, Sorenson JA, Guyer DR, Orlock DA . Digital indocyanine green videoangiography and choroidal neovascularization. Retina 1992; 12: 191–223.
Yuzawa M, Kawamura A, Matsui M . Clinical evaluation of indocyanine green video-angiography in the diagnosis of choroidal neovascular membrane associated with age-related macular degeneration. Eur J Ophthalmol 1992; 2: 115–121.
Lafaut BA, Aisenbrey S, Van den Broecke C, Bartz-Schmidt KU, Heimann K . Polypoidal choroidal vasculopathy pattern in age-related macular degeneration: a clinicopathologic correlation. Retina 2000; 20: 650–654.
Terasaki H, Miyake Y, Suzuki T, Nakamura M, Nagasaka T . Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol 2002; 86: 321–327.
Gass JDM . Update clininicopathologic classification of subretinal neovascularization. Am Soc Retina Special, Online Journal Vol. 3 2004.
Yoneya S, Saito T, Komatsu Y, Koyama I, Takahashi K, Duvoll-Young J . Binding properties of indocyanine green in human blood. Invest Ophthalmol Vis Sci 1998; 39: 1286–1290.
Guyer DR, Puliafito CA, Mones JM, Friedman E, Chang W, Verdooner SR . Digital indocyanine-green angiography in chorioretinal disorders. Ophthalmology 1992; 99: 287–291.
Imaizumi H, Takeda M . [Knobby-like choroidal neovascularization accompanied with retinal pigment epithelial detachment]. Nippon Ganka Gakkai Zasshi 1999; 103: 527–537.
Lim TH, Laude A, Tan CS . Polypoidal choroidal vasculopathy: an angiographic discussion. Eye 2010; 24: 483–490.
Otani A, Sasahara M, Yodoi Y, Aikawa H, Tamura H, Tsujikawa A et al. Indocyanine green angiography: guided photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 2007; 144: 7–14.
Maruko I, Iida T, Saito M, Nagayama D, Saito K . Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol 2007; 144: 15–22.
Sakurada Y, Kubota T, Imasawa M, Tsumura T, Mabuchi F, Tanabe N et al. Angiographic lesion size associated with LOC387715 A69S genotype in subfoveal polypoidal choroidal vasculopathy. Retina 2009; 29: 1522–1526.
Mori K, Horie-Inoue K, Gehlbach PL, Takita H, Kabasawa S, Kawasaki I et al. Phenotype and genotype characteristics of age-related macular degeneration in a Japanese population. Ophthalmology 2010; 117: 928–938.
Lee KY, Vithana EN, Mathur R, Yong VH, Yeo IY, Thalamuthu A et al. Association analysis of CFH, C2, BF, and HTRA1 gene polymorphisms in Chinese patients with polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 2008; 49: 2613–2619.
Kondo N, Honda S, Ishibashi K, Tsukahara Y, Negi A . LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population. Am J Ophthalmol 2007; 144: 608–612.
Gotoh N, Nakanishi H, Hayashi H, Yamada R, Otani A, Tsujikawa A et al. ARMS2 (LOC387715) variants in Japanese patients with exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Am J Ophthalmol 2009; 147: 1037–1041.
Lima LH, Schubert C, Ferrara DC, Merriam JE, Imamura Y, Freund KB et al. Three major loci involved in age-related macular degeneration are also associated with polypoidal choroidal vasculopathy. Ophthalmology 2010; 117: 1567–1570.
Kondo N, Honda S, Ishibashi K, Tsukahara Y, Negi A . Elastin gene polymorphisms in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 2008; 49: 1101–1105.
Okubo A, Arimura N, Abematsu N, Sakamoto T . Predictable signs of benign course of polypoidal choroidal vasculopathy: based upon the long-term observation of non-treated eyes. Acta Ophthalmol 2010; 88: e107–e114.
Okubo A, Hirakawa M, Ito M, Sameshima M, Sakamoto T . Clinical features of early and late stage polypoidal choroidal vasculopathy characterized by lesion size and disease duration. Graefes Arch Clin Exp Ophthalmol 2008; 246: 491–499.
Acknowledgements
This study was supported in part by the Japan Society for the Promotion of Science (JSPS), Tokyo, Japan (Grant-in-Aid for Scientific Research, no. 21592256), and the Japan National Society for the Prevention of Blindness, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tsujikawa, A., Ojima, Y., Yamashiro, K. et al. Development of polypoidal lesions in age-related macular degeneration. Eye 25, 481–488 (2011). https://doi.org/10.1038/eye.2010.232
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.232