Abstract
Aim
The aim of this study is to evaluate the long-term efficacy of intravitreal bevacizumab (IVB) for eyes with non-proliferative idiopathic macular telangiectasia type 2 (IMT2) and acute vision loss.
Methods
In this interventional case series, treatment-naive eyes of 13 consecutive patients with IMT2 were included. Eyes with a recent onset of visual loss were treated with 0.04 ml IVB (n=7). Fellow eyes and eyes of patients without disease progression served as control group (CG) (n=12). Follow-up examinations included ophthalmoscopy, best-corrected visual acuity (BCVA), optical coherence tomography, and fluorescein angiography (FA).
Results
Mean follow-up time was 32±5.7 months in the treatment group (TG) (n=7) vs 29±8.8 months in the CG (n=16). Mean BCVA increased from logMAR 0.47±0.32 at baseline to logMAR 0.33±0.31 (P=0.21) at the last visit in the TG and decreased from logMAR 0.25±0.39 to logMAR 0.30±0.40 in the CG (P=0.17). All patients in the TG showed stabilisation or improvement in vision in Snellen lines in contrast to the CG (χ2-test P=0.04). Patients received on average 2.3±1.3 IVB injections. Mean central millimetre thickness in TG and CG was 260±83 and 201±32 μm at baseline vs 237±69 and 199±29 μm at the last visit, respectively (P=0.23 and 0.77). FA revealed a significant decrease of the juxtafoveal staining size at month 3 (P= 0.004) and a slight reduction at the last visit (P= 0.11) in the TG.
Conclusion
Despite an overall moderate effect of IVB treatment, individual patients experience a marked functional and morphological long-term benefit.
Similar content being viewed by others
Introduction
Idiopathic macular telangiectasia type 2 (IMT2) is a rare disease of the macular capillaries associated with a concomitant progressive atrophy of the neural and glial tissue.1 IMT2 affects both eyes in 98% of patients and causes a decrease in visual acuity as well as metamorphopsia symptomatic around the fifth decade of life.2, 3 Optical coherence tomography (OCT) typically shows intraretinal cystoid cavities, disruptions or loss of outer retinal layers, and retinal thinning.1, 4, 5
The treatment effect of laser photocoagulation, photodynamic therapy, intravitreal triamcinolon, and retinal surgery are limited, and none of these treatment modalities have yet been accepted as a gold standard. Vascular endothelial-derived growth factor (VEGF) is supposed to have a role in the pathogenesis and natural course of IMT2. Recent interventional case series showed significant short-term improvement in visual acuity and leakage in both non-proliferative and proliferative IMT2 following intravitreal treatment with anti-VEGF.6, 7 However, little is known about the duration of this significant treatment effect.
The aim of this study was to evaluate the functional and morphological treatment effects of intravitreal bevacizumab (IVB) in eyes with progressive but non-proliferative IMT2 in a standardised way over an extended follow-up of 30 months. In addition, an untreated control group (CG) of eyes with silent IMT2 was included following the same evaluation procedures to provide a comparison with the natural course with slowly progressive visual and anatomical decline.
Materials and methods
In this interventional retrospective clinical study, thirteen consecutive patients (seven females, six males) with bilateral non-proliferative IMT2 were included. Seven eyes of six patients, who had a documented recent onset of visual loss received intravitreal anti-VEGF treatment (three females, three males). The quiescent treatment-naive fellow eyes of five patients and treatment-naive eyes of patients without recent vision loss served as CG (n=16). Patients with other retinal diseases such as epiretinal membranes, a history of vitreoretinal surgery, or pretreatment of any type were excluded from the study. The study adhered to the tenets of the Declaration of Helsinki. Informed consent for observation or treatment was obtained from every patient in both groups. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed up during this research.
All patients who received anti-VEGF treatment had to sign an informed consent describing the off-label use of IVB for the treatment of IMT2 and its potential risks. The intravitreal injection of 0.04 ml (1 mg) bevacizumab was performed following the recommended standard protocol under sterile conditions. The decision for postoperative treatment consisted of dexamethason and gentamicinsulfat eye drops (Dexagenta POS eye drops, Croma Pharma, Leobendorf, Austria) four times a day for 4 days. The decision for re-treatment with bevacizumab was based on visual acuity measurements and recurrence of central macular thickening and cyst formation by OCT. Re-treatments were performed, if more than 1 Snellen line visual acuity impairment and increase in macular thickness compared with previous follow-ups were observed.
Examinations at baseline and at each follow-up time included best-corrected visual acuity (BCVA), ophthalmoscopy, and OCT. Patients were examined at baseline, months 1, 3, 6, and 12, and thereafter in 6-month intervals. Fluorescein angiography (FA) was performed at baseline, at the end of follow-up, and if indicated during the study, for example, when a substantial increase in central millimetre thickness (CMT) had occurred.
OCT measurements were performed using conventional time-domain OCT (Carl Zeiss Meditec, Jena, Germany). Six cross-sectional line scans were taken and retinal thickness of all nine fields (CMT and fields 2–9 (F2–9)) was displayed on an Early Treatment Diabetic Study (ETDRS) grid. Retinal thickness and retinal volume (RV) was calculated using the standard OCT software (version 4.0.1 0056, Carl Zeiss Meditec).
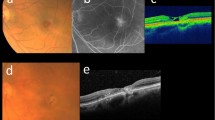
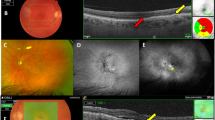
Presence of intraretinal cavities was evaluated in the horizontal and vertical scan of each eye at baseline and at the last visit and graded into three grades: grade 1 (diameter, 50–100 μm), grade 2 (diameter, 100–300 μm), and grade 3 (diameter, >300 μm). The documented error was defined as the highest grade present in either scan direction. Eyes with lamellar holes at baseline were excluded from this analysis.
For FA, a Heidelberg Retina Angiograph II (HRA II, Heidelberg Engineering, Heidelberg, Germany) was used. The size of early-stage (1 min) and late-stage (10 min) staining hyperfluorescent areas were assessed using the standard HRA software.
Statistical analysis
Statistical analysis was performed using Microsoft Excel and SPSS 16.0 software (SPSS Inc, Chicago, IL, USA). The outcome parameters of this study were changes in visual acuity, central retinal thickness, RV, and FA leakage size. In addition, ultrastructural morphological features (presence of intraretinal cavities) were evaluated. For statistical analysis, the BCVA was converted from Snellen decimal to the logarithm of the minimal angle of resolution (logMAR). The level of statistical significance was calculated using repeated ANOVA, paired t-test, and χ2-test. P-values of <0.05 were considered to be statistically significant.
Results
The mean age of the study patients was 59±9.3 years (n=13). Mean follow-up time was 32±5.7 months in the treatment group (TG) (n=7) vs 29±8.8 months in the CG (n=16). The descriptive data of the patients are illustrated in Table 1.
Visual acuity
Mean BCVA in the TG was logMAR 0.47±0.32 (range, 0.12–1; median, 0.40) at baseline. At 1 month after the first injection, overall mean BCVA of the entire group improved slightly to logMAR 0.30±0.25 (range, 0.05–0.40; median, 0.30, P=0.09). Stabilisation of BCVA until month 12 was observed (mean logMAR, 0.29±0.20; range, 0.10–0.40; median, 0.22; P=0.09). Again, at the last follow-up visit, after a mean of 30 months, no statistically significant difference was observed with regard to BCVA compared with baseline (mean logMAR, 0.33±0.31; range, 0.15–1; median, 0.15; P=0.21). Figure 1 illustrates the course of BCVA and CMT.
Patients received on average 2.3±1.3 IVB injections. Two patients received a single injection; three patients received two; and two patients received a total of four injections. The mean duration between the re-injections was 2.2±1.6 months.
Mean BCVA in the CG was logMAR 0.30±0.40 (range, 0.0–1.5; median, 0.10). BCVA deteriorated with slight fluctuations and was reduced to logMAR 0.30±0.40 (range, 0.05–1.70; mean, 0.23, P=0.17) at the last visit. There was no significant improvement of BCVA at any follow-up visit.
A statistically significant difference was found between both study groups regarding BCVA change in Snellen lines. In comparison with baseline, BCVA showed an improvement of ⩾1 Snellen line in four eyes and stabilisation in three eyes of the TG (n=7), whereas in untreated control eyes (n=16), BCVA improved to ⩾1 Snellen line in only two eyes, remained stable in four eyes, and worsened in 10 eyes with ⩾1 Snellen line at the last follow-up visit (χ2-test, P=0.04).
Morphological outcomes
Mean CMT in the TG was 260±83 μm at baseline. At 1 month after the first injection CMT, all inner fields (F2–5) and F9 showed a remarkable decrease in mean retinal thickness (P>0.05). Mean CMT showed the greatest decrease from 260 to 202±37 μm. Mean RV declined modestly from 7.29±0.68 to 7.04±0.64 mm3 at month 1 (P=0.15). At the last follow-up visit, the overall decrease in retinal thickness from month 1 was diminished in CMT and in all inner fields (F2–5). Average retinal thickness values were, however, uniformly at a lower level than at baseline in those fields. CMT averaged with 237±69 μm; RV at the last visit (7.06±0.67 mm3) was quite similar to month 1 (7.04±0.64 mm3). Figure 2 summarises the changes in retinal thickness in CMT and F2–9 for both groups over the time of the entire study. In the TG, stage 3, 2, and 1 intraretinal cavities were observed in two, one, and one eyes at baseline, and in three, 0, and one eyes at the last follow-up, respectively. A characteristic example of the morphological changes under therapy is illustrated in Figure 2.
In the CG, mean CMT was 201±32 μm and RV averaged 6.81±0.52 mm3 at baseline. At the last visit, a minimal decline in retinal thickness in all fields (CMT, F2–9) varying between 2.5 and 13 μm was assessed (Figure 2). Mean CMT and RV had slightly decreased to 199±29 and 6.69±0.49 mm3, respectively (P>0.05). There was no significant change in retinal thickness in any field at any visit. In the CG, stage 3, 2, and 1 intraretinal cavities were observed in three, four, and two eyes at baseline, and in three, four, and two eyes at the last follow-up time, respectively.
Angiographic outcomes
The diagnosis of non-proliferative IMT2 was confirmed by FA in all eyes at baseline. In the TG, after the first injection of bevacizumab, the mean size of active vascular staining hyperfluorescent area decreased significantly within a period of 3 months in both early-stage FA (from 1.80±1.35 to 0.19±0.14 mm2; P=0.045) and late-stage FA images (from 2.99±1.31 to 0.43±0.18 mm2; P=0.004). The size of staining hyperfluorescent area remained on a decreased level in comparison with baseline. At the last follow-up visit, the mean early-stage staining hyperfluorescent area measured 1.61±1.5 mm2 and mean late-stage staining hyperfluorescent area was 2.52±1.35 mm2 (P=0.12 and 0.11, respectively).
In the CG, the area of early-stage staining hyperfluorescent area remained quite unchanged between baseline and the last follow-up visit (1.03±1.24 and 1.13±1.05 mm2, respectively; P=0.43). The size of late-stage staining hyperfluorescent area increased from 1.60±1.56 to 1.75±1.52 mm2 (n=8; P=0.36).
Complication or adverse effects
No IVB-associated complication or adverse effects were observed. Cataract surgery was only performed in one eye during the entire study.
Discussion
In this study, we analysed the functional and morphological long-term effects of IVB in symptomatic eyes with non-proliferative, treatment-naive IMT2, in comparison with the natural course of disease progression over a follow-up of 30 months.
Our results appear to outline perspectives of IVB treatment in IMT2 in an exemplary manner. As IMT2 becomes symptomatic, an acute BCVA loss occurs and this functional change is associated with a morphological alteration, that is, increase in CMT due to the formation of intraretinal cysts. As functional loss and retinal thickening is only moderate, both parameters will not reach statistically significant levels as mean values over the entire IMT2 population. However, as the variability between individual patients is substantial, selected patients will benefit at a significant level. Again, the therapeutic benefit in terms of function and morphology is rather small, for example, compared with the level of change in neovascular age-related macular degeneration, and will not reach significance over the entire average group. A defined proportion of patients with acute disease will, however, clearly benefit with a marked gain in vision and a measurable reduction in cystic retinal swelling. Moreover, the therapeutic gain will be maintained during long-term follow-up and no adverse events or acceleration of disease activity is noted compared with the natural course in an untreated CG.
In our TG, the number of patients with stabilisation and improvement of BCVA in Snellen lines after 30 months was significantly higher compared with the CG. Furthermore, angiographic outcomes presented a statistically significant short-term decrease of the staining hyperfluorescent area.
Improvement in visual acuity as well as morphological and angiographic parameters demonstrated a positive long-term trend, with selected patients presenting a superior benefit.
The CG showed a slow progression in visual decline similar to previous reports by Gass and Blodi.2 Especially the late stage of IMT2 in the natural course is reported to have a poor prognosis because of the development of retinal and epiretinal scars.8 In our study, one patient in the TG developed a lamellar hole. The foveal atrophic changes within the neurosensory retina in IMT2 may predispose the development of macular holes.9 The treatment with IVB might accelerate this process by its impact on the cystic changes.
Macular telangiectasia may be a more common condition than previously assumed. The macular alterations in asymptomatic patients can be so subtle that they can only be detected with recently available imaging technologies, such as spectral-domain OCT.10
The pathogenesis of IMT2 is controversial1 and the exact role of VEGF is not completely understood. Green et al11 postulated that proliferation of the basement membrane leads to thickening of the wall of retinal capillaries in eyes with IMT2. This phenomenon may lead to hypoxia-induced VEGF release. Positive results of clinical trials using anti-VEGF for the treatment of IMT2 seem to confirm the theory of pathologically increased VEGF levels in eyes with active disease.6, 7, 12 Nevertheless, documented measurements of anti-VEGF levels in eyes with IMT2 have not been published yet.
In a study of Charbel Issa et al12, visual acuity increased significantly 1 month after the first treatment and maintained significantly improved even over a period of 18 months in eyes with non-proliferative IMT2 using repeated bevacizumab injections. The authors also reported significant quantitative long-term changes in FA and OCT. Contrarily, Kovach and Rosenfeld13 did not observe any significant change in BCVA or retinal thickness at any visit after treatment with IVB over the time of 18 months in non-proliferative IMT2. In our study, the angiographic staining hyperfluorescent area decreased significantly within the first 3 months after the first IVB injection. At the last follow-up visit, the staining hyperfluorescent area was slightly increased, but still below baseline levels. In untreated eyes, however, the staining hyperfluorescent area was slightly increased in comparison with baseline.
Comparing mean retinal thickness decrease between baseline and the last follow-up visit of both groups, we could not observe any significant difference in any field of the ETDRS ring. There was only a tendency towards a different distribution: Treated eyes showed a greater mean decrease of retinal thickness in CMT and the inner fields F4–5, whereas the control group revealed a larger mean decrease especially in the outer fields (F7–9).
The limitations of this study include the small sample size and the lack of a standardised randomisation. However, we identified the changes in terms of morphological, angiographic, and functional parameters before and after IVB compared with natural course in great detail and over a long follow-up. Another limitation of this study may be that the re-treatment was based on clinical, as well as morphological outcomes, and no loading dose was given a priori. Our results may offer a realistic view on the treatment of IMT2 and provide a rationale for treatment with IVB. In summary, IVB seems to be a safe therapy with a stabilising functional and morphologic–angiographic long-term effect in eyes with IMT2 compared with the natural course. Prospective, randomised clinical trials with a larger sample size are clearly needed to confirm the hypothesis of a moderate, but maintained benefit without the risk of acceleration in the course of IMT2.

References
Gaudric A, Ducos de Lahitte G, Cohen SY, Massin P, Haouchine B . Optical coherence tomography in group 2A idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol 2006; 124: 1410–1419.
Gass JD, Blodi BA . Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology 1993; 100: 1536–1546.
Gass JD, Oyakawa RT . Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol 1982; 100: 769–780.
Paunescu LA, Ko TH, Duker JS, Chan A, Drexler W, Schuman JS et al. Idiopathic juxtafoveal retinal telangiectasis: new findings by ultrahigh-resolution optical coherence tomography. Ophthalmology 2006; 113: 48–57.
Cohen SM, Cohen ML, El-Jabali F, Pautler SE . Optical coherence tomography findings in nonproliferative group 2a idiopathic juxtafoveal retinal telangiectasis. Retina 2007; 27: 59–66.
Charbel Issa P, Holz FG, Scholl HP . Findings in fluorescein angiography and optical coherence tomography after intravitreal bevacizumab in type 2 idiopathic macular telangiectasia. Ophthalmology 2007; 114: 1736–1742.
Mandal S, Venkatesh P, Abbas Z, Vohra R, Garg S . Intravitreal bevacizumab (Avastin) for subretinal neovascularization secondary to type 2A idiopathic juxtafoveal telangiectasia. Graefes Arch Clin Exp Ophthalmol 2007; 245: 1825–1829.
Watzke RC, Klein ML, Folk JC, Farmer SG, Munsen RS, Champfer RJ et al. Long-term juxtafoveal retinal telangiectasia. Retina 2005; 25: 727–735.
Charbel Issa P, Scholl HP, Gaudric A, Massin P, Kreiger AE, Schwartz S et al. Macular full-thickness and lamellar holes in association with type 2 idiopathic macular telangiectasia. Eye 2009; 23: 435–441.
Gillies MC, Zhu M, Chew E, Barthelmes D, Hughes E, Ali H et al. Familial asymptomatic macular telangiectasia type 2. Ophthalmology 2009; 116: 2422–2429.
Green WR, Quigley HA, de la Cruz Z, Cohen B . Parafoveal retinal telangiectasis: light and electron microscopy studies. 1977. Retina 2005; 25: 162–170.
Charbel Issa P, Finger RP, Holz FG, Scholl HP . Eighteen-month follow-up of intravitreal bevacizumab in type 2 idiopathic macular telangiectasia. Br J Ophthalmol 2008; 92: 941–945.
Kovach JL, Rosenfeld PJ . Bevacizumab (avastin) therapy for idiopathic macular telangiectasia type II. Retina 2009; 29: 27–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work was presented at the DOG-Congress 2009 in Leipzig.
Rights and permissions
About this article
Cite this article
Matt, G., Sacu, S., Ahlers, C. et al. Thirty-month follow-up after intravitreal bevacizumab in progressive idiopathic macular telangiectasia type 2. Eye 24, 1535–1542 (2010). https://doi.org/10.1038/eye.2010.113
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.113
Keywords
This article is cited by
-
Comparison of anatomical and visual outcomes following different anti-vascular endothelial growth factor treatments in subretinal neovascular membrane secondary to type 2 proliferative macular telangiectasia
Graefe's Archive for Clinical and Experimental Ophthalmology (2020)
-
Management of Idiopathic Macular Telangiectasia Type 2
Ophthalmology and Therapy (2019)
-
Therapeutische Ansätze bei makulären Teleangiektasien Typ 2
Der Ophthalmologe (2014)
-
Comparison of observation, intravitreal bevacizumab, or pars plana vitrectomy for non-proliferative type 2 idiopathic macular telangiectasia
Graefe's Archive for Clinical and Experimental Ophthalmology (2013)
-
Long-term course in type 2 idiopathic macular telangiectasia
Graefe's Archive for Clinical and Experimental Ophthalmology (2013)