Abstract
Purpose
To evaluate visual outcomes in patients with neovascular age-related macular degeneration (NV-AMD) who were treated with pegaptanib sodium in European clinical ophthalmology practices.
Methods
Thirteen centres in eight European countries participated in this retrospective study. Medical records for patients with any angiographic subtype of subfoveal choroidal neovascularisation secondary to NV-AMD with visual acuities (study eye) of 20/40–20/320 treated with 0.3 mg pegaptanib as first-line treatment and with at least 24 weeks of follow-up were identified. Anonymised data reflecting at least 24 and up to 54 weeks of follow-up were recorded. Primary end points were visual acuity outcomes at weeks 24 and 54 compared with those reported at week 54 in the vascular endothelial growth factor (VEGF) Inhibition Study in Ocular Neovascularisation (VISION) trial.
Results
In all, 253 patients were followed for at least 24 weeks; 62 patients completed 54 weeks of follow-up. A mean of 4.4 (SD, 1.8) pegaptanib injections were administered through 24 weeks. Compared with the VISION trial, the European experience showed that >90% of patients in the current cohort lost <15 letters from baseline at both time points compared with 70% in the VISION trial at 54 weeks. Pegaptanib was well tolerated with no reported cases of endophthalmitis, traumatic cataract, or iatrogenic retinal detachment.
Conclusions
Pegaptanib was found to stabilise vision in a greater percentage of patients and produced greater overall visual improvement in this group of treatment-naive patients with NV-AMD compared with outcomes reported in the VISION trial; however, interpretation of these results should be tempered given the differences in design between this retrospective study and the prospective controlled trial.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is the leading cause of vision loss in the Western world, with ∼90% of severe vision loss attributable to the neovascular form.1 Until recently, treatment options for neovascular AMD (NV-AMD) were limited to thermal laser photocoagulation and photodynamic therapy (PDT) with verteporfin (Visudyne, Novartis International AG, Basel, Switzerland).2 In observational studies with PDT, visual outcomes were similar to those obtained in randomised clinical trials despite the use of fewer treatments.3
An improved understanding of the role of vascular endothelial growth factor (VEGF) in the pathogenesis of choroidal neovascular membranes (CNV) has led to the use of intravitreally administered anti-VEGF antibodies (ranibizumab and bevacizumab) and an aptamer (pegaptanib sodium) in the treatment of these lesions.4, 5, 6, 7 These new therapies have surpassed previous treatments in both efficacy and safety and are now the standard of care for NV-AMD.
The phase III VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial8 showed the safety and efficacy of six weekly pegaptanib injections (Macugen, Pfizer Inc., New York, NY, USA), a selective VEGF165 inhibitor, in eyes with NV-AMD. In this randomised, sham-controlled trial, vision was stabilised (defined as a loss of <15 letters on an Early Treatment Diabetic Retinopathy Study (ETDRS) logMAR chart) in 70% of eyes (treatment responders) and improved (defined as a gain of ⩾15 letters) in 6% of eyes with the 0.3-mg dose of pegaptanib at the end of 12 months. The European Medicines Agency9 approved the use of pegaptanib in 2006. Subsequently, many retinal specialists have treated patients with NV-AMD with this new agent. The aim of this retrospective study was to evaluate visual outcomes in patients with NV-AMD who were treated with pegaptanib in clinical ophthalmology practices in Europe and to compare results with those found in the phase III pivotal pegaptanib trial.8
Materials and methods
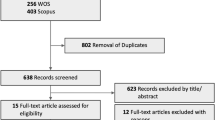
In all, 13 centres in eight European countries (Czech Republic, Germany, Ireland, Italy, Portugal, Spain, Turkey, and the United Kingdom) participated in the study. Medical records for consecutive patients with any angiographic subtype of subfoveal CNV secondary to NV-AMD with best-corrected visual acuities in the study eye of 20/40–20/320 who received 0.3 mg pegaptanib as first-line treatment and who had at least 24 weeks of follow-up were identified. Included patients were examined every 6 weeks and received 0.3 mg of intravitreal pegaptanib at the clinician's discretion. A combination of visual acuity, optical coherence tomography, and fundus fluorescein angiography was used to assess CNV activity and the need for retreatment. Patients were excluded if they were treated for NV-AMD with any agent other than pegaptanib before or during the study period.
Anonymised data reflecting at least 24 and up to 54 weeks of follow-up were recorded on Excel spread sheets. Data elements were type of membrane, lesion size, visual acuity at baseline and at 6-week intervals until final follow-up, and ocular or systemic side effects. Snellen visual acuity data were converted to equivalent letter scores from a standard 2-m ETDRS chart.
The primary end points were visual acuity outcomes at weeks 24 and 54 in patients remaining on therapy after 24 weeks compared with those reported at week 54 in the VISION trial,8 in particular, with regard to stabilisation of vision (loss of ⩽15 letters) in at least 70% of patients and vision improvement (gain of ⩾15 letters) in at least 6% of patients. Missing visual acuity data were imputed using the last observation carried forward method; baseline data were not carried forward, and data were carried forward only for one post-baseline visit.
Results
Baseline patient characteristics
Experience with pegaptanib from 1 July 2006 to 31 December 2007 was summarised. In all, 253 patients were followed for at least 24 weeks, and 62 patients completed 54 weeks of follow-up. Table 1 summarises baseline visual acuity across lesion subtypes. Numbers of patients with documented lesion size (according to greatest linear diameter) varied across time points. At 24 weeks, lesion size was documented for 208 patients: 74,<2500 μm; 77, 2500–4500 μm; and 57,>4500 μm. At week 54, lesion size was documented for 62 patients: 16,<2500 μm; 32, 2500–4500 μm; and 14,>4500 μm.
Pegaptanib administration
Although the timing and frequency of pegaptanib injections were at the clinician's discretion, it is notable that the first three injections were administered at 6-week intervals in all but five patients. Across the centres, a mean of 4.4 (SD, 1.8) pegaptanib injections were administered through 24 weeks. An average of 4.8 (SD, 1.9) injections were given through week 54 (range, 2–9); four patients had seven or more injections and forty had five or fewer. Mean number of injections did not vary substantially across lesion subtypes (Figure 1a and b).
Comparison of vision-related end points: the European experience vs the VISION trial
Table 2 presents the vision-related end points from this European experience and those of the VISION trial8 at both weeks 24 and 54. In particular, more than 90% of patients in the current cohort lost <15 letters from baseline at both time points; 70% of patients lost <15 letters in the VISION trial8 at 54 weeks. Gains of ⩾15 letters were achieved by 10.7% of patients at week 24 and by 4.8% at week 54, whereas 6% were reported at week 54 in the VISION trial. A loss of ⩾30 letters was noted in only 1.2% of patients at week 24 and in no patient at week 54 in the European cohort; 10% of patients in the VISION trial had a loss of ⩾30 letters at week 54. Proportions of patients with visual acuity ⩽20/200 were 11.9 and 9.7% at weeks 24 and 54, respectively, in the European cohort and 38% at week 54 in the VISION trial.
Visual acuity change by lesion subtype, size, and baseline visual acuity
Figure 2 shows the mean change in visual acuity from baseline for the 62 patients who completed 54 weeks of follow-up. Mean vision gains at 24 weeks were not sustained at 54 weeks among those with minimally classic or predominantly classic lesions. The difference in mean visual acuity change from baseline was not statistically significant across groups (analysis of variance, P=0.6).
In general, outcomes did not vary substantially with lesion size at any time point, although smaller lesions showed a slight trend towards greater improvement in visual acuity from 24 to 48 weeks compared with lesions >4500 μm (Figure 3).
Baseline visual acuity was dichotomised as ⩾54 letters (n=91) and <54 letters (n=162). The mean change in vision at 24 weeks in eyes with better baseline vision was +2.28 (SD, 10) vs −1.6 (SD, 12) letters among those with poorer baseline vision. A total of 21 patients in the group with better baseline visual acuity and 41 of those with poorer baseline vision completed 54 weeks of follow-up; at that time point, mean changes in visual acuity were −1.01 (SD, 12) letters and −2.1 (SD, 8) letters, respectively.
Adverse events
Pegaptanib was well tolerated. There were no reported cases of endophthalmitis, traumatic cataract, or iatrogenic retinal detachment or cases of thromboembolic, cerebrovascular events, or myocardial infarctions.
Discussion
In the VISION trial,8 after 54 weeks of pegaptanib treatment at 6-week intervals, 70% of patients with CNV had stabilisation of vision (lost <15 letters) and 6% had visual improvement (gained ⩾15 letters of vision). Although our real-life experience found that pegaptanib generally resulted in better visual outcomes, that is, 93 and 92% of patients lost <15 letters at 24 and 54 weeks, respectively; these differences in outcomes could be due to the lack of standardisation of treatment and retreatment eligibility criteria and visual acuity measurement techniques, as well as the lack of blinding in our study.
Although the majority of patients enrolled in the VISION trial8 had previously treated lesions, a post hoc subgroup analysis10 of the VISION data set explored the effect of pegaptanib on those patients who were treatment-naive (no previous PDT/thermal laser photocoagulation) and who had early disease defined as lesion size <2 disc areas, a baseline visual acuity ⩾54 letters, and an absence of scarring/atrophy. At week 54, visual acuity in 26 of 34 (76%) of the pegaptanib-treated subgroup had stabilised and 12 of 34 (35%) had maintained or gained vision. This issue is being further examined in the ongoing PERSPECTIVES trial; similar criteria for classifying lesions as early stage are being used but expanded to include those measuring ⩽12 disc areas, providing that they are assessed as <50% classic component by fluorescein angiography and without lipid exudation, retinal epithelial detachment, or subfoveal haemorrhage. Our cohort did not reveal differences in outcomes across lesion sizes, possibly reflecting the small numbers of patients in the lesion subtype categories who completed 54 weeks of follow-up. However, patients with smaller lesions did show a slight trend towards greater improvement in visual acuity from 24 to 48 weeks compared with those having lesions >4500 μm. Findings of small retrospective case series of patients treated with pegaptanib in the United States, published after the drug was introduced in January 2005, are consistent with the expectation that earlier treatment produces better clinical outcomes.11, 12
In this European cohort, a large proportion of patients had occult CNV. The exploratory analysis of VISION trial8 patients with earlier-stage lesions also found better responses for treatment-naive occult lesions with 20% of eyes with active occult disease gaining ⩾3 lines of vision.10 Similarly, in a review of 90 patients with newly diagnosed NV-AMD who were observed for a minimum of 6 months (mean, 9.1±2 months), pegaptanib as primary therapy provided a 90% rate of improvement or stabilisation of vision;11 80% (72/90) of lesions were classified as occult.
Although pegaptanib was administered at 6-week intervals throughout the 48 weeks in the VISION trial,8 European investigators participating in this study administered pegaptanib as needed (prn) at their discretion. At week 24, a mean of 4.4 injections had been administered with an average of 4.8 injections administered through week 54. This relatively small increase indicates that the majority of patients did not receive injections in the second half of the year, a fact that may explain the decrease in visual acuity between weeks 24 and 54. The VISION trial8 showed that patients randomised to discontinue pegaptanib after 1 year of treatment were at greater risk of vision loss than those continuing treatment.13 Similarly, the PIER study,14 which evaluated an every-3-month dosing regimen for ranibizumab in patients with subfoveal CNV, revealed that visual acuity tended to diminish when compared with the standard every-4-week dosage regimen recommended for this agent. The prn schedule in our cohort was based on the investigator's discretion. Not all treatments were guided by retreatment criteria as defined in the PrONTO study.15 The prn schedule is a rather new treatment strategy and with experience, investigators have noted that a regimen of very aggressive optical coherence tomography and visual acuity guidance is required to sustain the initial gain in vision after ranibizumab. This premise also may apply to pegaptanib.
As in the VISION trial,8, 13 pegaptanib was well tolerated. In the setting of NV-AMD, the safety of pegaptanib has now been validated over 4 years and in some cases in patients receiving >35 injections without the appearance of ocular or systemic safety signals16, 17 that have been reported with the use of the pan-VEGF agents. Although currently available data do not allow for a direct comparison of the safety of the three VEGF antagonists, of particular note is the SAILOR trial, a dedicated study comparing two doses of ranibizumab, which found a tendency towards a higher incidence of stroke recurrence among patients with a history of stroke (9.6% in the 0.5-mg group compared with 2.7% in the 0.3 mg group); the difference, however, was not statistically significant (Boyer D et al.,18 safety in previously treated and newly diagnosed patients with neovascular age-related macular degeneration (AMD): the SAILOR study. Presented at Bascom Palmer Eye Institute Angiogenesis, Exudation and Degeneration Meeting, 22–23 February 2008, Key Biscayne, FL, USA).
Limitations of this study include its retrospective and uncontrolled design and especially the fact that criteria for retreatment were not standardised across sites. In addition, visual acuity measurement methods varied, and Snellen measurements were converted to ETDRS letters. Finally, data on central macular thickness were not routinely available, but would have provided an additional basis for comparing our findings with those of the landmark studies.
Of note, although the outcomes found with pegaptanib sodium in the treatment of treatment-naive NV-AMD in this real-life experience showed more improvement than that reported in the VISION study,8 the differences in design between this retrospective study and the prospective controlled VISION trial limit the comparability of results. Until studies directly comparing ranibizumab, bevacizumab, and pegaptanib are undertaken, pegaptanib may be optimally warranted for patients who are not candidates for pan-VEGF agents on a long-term basis.

References
Ferris 3rd FL, Fine SL, Hyman L . Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984; 102: 1640–1642.
Ahmadi MA, Lim JI . Pharmacotherapy of age-related macular degeneration. Expert Opin Pharmacother 2008; 9: 3045–3052.
Murjaneh S, Garcia-Finana M, Mahmood S, Lenfestey PM, Taylor SA, Pearce IA et al. Observational prospective study of the effectiveness in routine clinical practice of verteporfin photodynamic therapy in patients with neovascular age-related macular degeneration. Br J Ophthalmol 2009; 93(4): 468–473.
Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol 2007; 144: 850–857.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431.
VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trial Group D’Amico DJ, Masonson HN, Patel M, Adamis AP, Cunningham Jr ET, Guyer DR et al. Pegaptanib sodium for neovascular age-related macular degeneration: two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology 2006; 113: 992–1001.
Pedersen KB, Sjølie AK, Møller F . Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration in treatment-naive patients. Acta Ophthalmol 2008, e-pub ahead of print 16 December 2008.
Gragoudas ES, Adamis AP, Cunningham Jr ET, Feinsod M, Guyer DR . Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004; 351: 2805–2816.
European Medicines Agency. EPARs for authorised medicinal products for human use: Macugen. Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/macugen/macugen.htm. Accessed on 23 January 23 2009.
Gonzales CR . Enhanced efficacy associated with early treatment of neovascular age-related macular degeneration with pegaptanib sodium: an exploratory analysis. Retina 2005; 25: 815–827.
Quiram PA, Hassan TS, Williams GA . Treatment of naive lesions in neovascular age-related macular degeneration with pegaptanib. Retina 2007; 27: 851–856.
Ehlers JP, Fintak DR, Gupta OP, Regillo CD, Fineman MS, Ho AC . Pegaptanib for choroidal neovascularization in treatment-naive exudative age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2007; 38: 371–377.
VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trial Group. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 2006; 113: 1508–1521.
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol 2008; 145: 239–248.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007; 143: 679–680.
Singerman LJ, Masonson H, Patel M, Adamis AP, Buggage R, Cunningham Jr E et al. Pegaptanib sodium for neovascular age-related macular degeneration: third-year safety results of the VISION trail. Br J Ophthalmol 2008; 92: 1606–1611.
Marcus DM . Four-year safety of pegaptanib sodium in neovascular age-related macular degeneration (AMD): results of the VISION trial. Invest Ophthalmol Vis Sci 2008; 49, E-Abstract 5069.
Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG . A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology 2009; 116(9): 1731–1739.
Acknowledgements
This paper was presented in part at the Annual Meeting of the Association for Research in Vision and Ophthalmology, 27 April–1 May 2008, Fort Lauderdale, Florida, USA, and at the European Society of Retina Specialists 8th Congress, 22–25 May 2008, Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All authors have received travel or research grants from Pfizer Inc., New York, NY, USA.
Rights and permissions
About this article
Cite this article
Sivaprasad, S., Hykin, P., Saeed, A. et al. Intravitreal pegaptanib sodium for choroidal neovascularisation secondary to age-related macular degeneration: Pan-European experience. Eye 24, 793–798 (2010). https://doi.org/10.1038/eye.2009.232
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.232