Abstract
Purpose
To define molecular and ophthalmic features of a rare phenotype in autosomal dominant (ad) retinitis pigmentosa (RP)
Methods
A 32-year-old woman (proband) with adRP and the low-frequency damped electroretinographic (ERG) wavelet phenotype and her mother were studied with optical coherence tomography (OCT), chromatic perimetry and ERG. A previously reported adRP patient with this ERG phenotype (Lam et al) was also studied with OCT. Genotype in the two families was determined with DNA sequencing.
Results
ERGs from the proband were identical to those reported previously. Chromatic perimetry and ERG stimulus intensity series indicated that there can be severely reduced rod function in addition to substantial cone dysfunction. A heterozygous deletion in peripherin/RDS (Met152del3 atGAA) was present in the patient and the affected mother. There were foveal cystoid changes and pericentral splitting of the inner nuclear layer. ONL thickness and vision tapered with eccentricity, and ‘blind’ regions without discernible ONL showed a thickened, delaminated inner retina. Similar OCT findings were present in the reported adRP patient with this ERG; the patient was heterozygous for a 4-bp deletion (Leu107del4 ctGAGT) in PRPF31.
Conclusions
The low-frequency damped ERG wavelet phenotype is genetically heterogeneous. Inner retinal structural abnormalities are also present in this rare disease expression.
Similar content being viewed by others
Case report
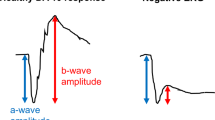
The 32-year-old proband presented with nyctalopia and decreasing visual acuity (VA). Her mother had maculopathy; a maternal aunt had pattern dystrophy (Figure 1a). The maternal grandfather had blindness of unknown cause. Institutional review board approval and informed consent were obtained. Best-corrected VA was 20/25 (+2.25), right, and 20/32 (+1.50), left. There were central cystoid changes, attenuated retinal vessels, bilateral optic nerve drusen, and depigmented-appearing periphery. ERGs showed a barely detectable rod b-wave, reduced a- and b-waves of the mixed cone-rod signal, and reduced and delayed cone responses (Figure 1b). The mixed ERG has low-frequency (20–25 Hz) damped oscillations (50–150 ms after stimulus onset).1 Faster oscillatory potentials were absent. Dark-adapted stimulus-intensity series (Figure 1c) shows an elevated rod b-wave threshold and the characteristic waveform at high intensities. Kinetic fields were full with V-4e, but constricted to I-4e. Chromatic static perimetry demonstrated pericentral-midperipheral rod and cone dysfunction with better-functioning peripheral field (Figure 1d).
(a) Pedigree, proband: arrow; filled symbols: affected; open symbols: unaffected. Stars, examined by the authors. (b) ISCEV standard ganzfeld ERGs from the proband and a normal subject (age: 31 years) obtained with a computer-based system (Espion, Diagnosys LLC, Littleton, MA, USA) with eye monitoring through a built-in infrared video system. Pupils were fully dilated with mydriatics for the recordings. Mixed cone-rod and cone ERGs are elicited with single white flashes (0.76 log cd.s.m−2); light-adapted, cone-mediated responses are online averages of 10–20 responses to the same stimulus. Thinner traces are additional responses to illustrate reproducibility. Digital filter is disabled and automatic artefact rejection is set at 1 mV. Vertical bars indicate stimulus onset. Calibrations are to the right and below waveforms. Arrows: damped wavelets. Rod b-waves are reduced (to 5% of mean normal); mixed ERGs have reduced a- and b-waves (to 20%); and cone ERGs (1 and 29 Hz) are reduced (to ⩽50%) with delayed timing. (c) ERGs elicited by increasing intensities of white light stimuli in the dark- and light-adapted states in the proband (right column) compared to a normal subject (left). (d) Kinetic perimetry (top) and chromatic static perimetry displayed as grayscale maps of rod (middle) and cone (bottom) sensitivity loss. Physiological blind spot is a black square at 12° in the temporal field. N, nasal; T, temporal; I, inferior; S, superior visual field.
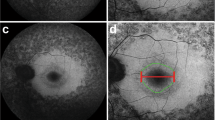
Optical coherence tomography (OCT) at age 32 showed central cystoid changes (Figure 2a). Inner nuclear layer (INL) had hyporeflectivity crossed by vertical bands of higher reflectivity (Figure 2a, arrow) in a schisis-like pattern. Outer nuclear layer thinned with eccentricity. At 3 mm temporal, retinal thickness is increased and normal lamination is lost. Serial OCTs demonstrate diminution of central cystoid but persistence of schisis-like changes. A vertical OCT shows central cystoid and, between 1 and 4 mm eccentric, INL splitting. There is no major visual disturbance in the central 2.5 mm: colocalized rod function is within 3 dB of normal lower limit and cone function is normal. Superior to 5 mm, retinal delamination is associated with no measurable vision (Figure 2b).
(a) Serial OCT3 images from the horizontal meridian of the proband (F1, PII-1) compared to a normal subject (top panel) and to an adRP patient with the same ERG abnormality (F2, PIV-1 from reference 1). White arrows point to regions with inner retinal separation. Insets: scan location on fundus schematic. Images are displayed with the logarithm of reflectivity mapped to a pseudocolor scale. F, fovea; N, nasal; T, temporal retina. Brackets defining outer nuclear layer (ONL) and inner retina (structures vitread to outer plexiform layer) are labelled (left edge of normal scan); black arrows point to photoreceptor inner/outer segments (IS/OS), and retinal pigment epithelium (RPE) layers. (b) Fourier-domain OCT images of >7 mm length of superior retina of F1, PII-1 (right panel), and a normal subject (left). Bars above the cross-sections indicate rod (blue bar; dark-adapted, 500-nm stimuli) and cone (red bar; light-adapted, 600-nm stimuli) sensitivity measured by chromatic static perimetry; dashed lines: normal mean minus 2SD. I, inferior; S, superior retina. Inset: scan location on a schematic of the fundus.
The proband's 60-year-old mother had long-standing central vision complaints. VA was 20/32 (right) and 20/20 (left). Central retinal pigment epithelium changes were present. Kinetic fields and ERGs were normal. There were no cystoid or schisis-like changes on OCT.
Patient F2, PIV-1 from the published study,1 had central cystoid, paracentral schisis-like changes, and abnormally laminated retina at greater eccentricities (Figure 2a, bottom panel).
The proband and her mother harboured a heterozygous 3-bp deletion (Met152del3 atGAA) in peripherin/RDS.3 In Family 2, PIII-2 and PIV-1,1 a novel 4-bp deletion (Leu107del4 ctGAGT) of the PRPF31 gene segregated with disease.
Comment
The low-frequency damped ERG phenotype is not genotype specific: there is association with mutations in peripherin/RDS or PRPF31. This ERG has now been noted in patients ranging in age from 7 to 32 years.1 Follow-up should clarify whether patients move from stages without wavelets into a wavelet phase and on to more advanced stages with non-detectable ERGs. We also report schisis-like INL changes in this rare disease expression; whether this relates to the pathophysiology is uncertain.
We speculate that the unusual ERG signal may be due to coincidence of two rare phenotypes in the same individual. Rare variations in normal retinal pathways4 could reduce feedback inhibition on inner retinal cells expressing oscillatory behaviour.5 Wavelets may result from unmasking of such oscillations when degeneration causes imbalance in the parallel signal streams traversing the retina.
References
Lam BL, Liu M, Hamasaki DI . Low-frequency damped electroretinographic wavelets in young asymptomatic patients with dominant retinitis pigmentosa: a new electroretinographic finding. Ophthalmology 1999; 106: 1109–1113.
Kennan A, Aherne A, Humphries P . Light in retinitis pigmentosa. Trends Genet 2005; 21: 103–110.
Weleber RG, Carr RE, Murphey WH, Sheffield VC, Stone EM . Phenotypic variation including retinitis pigmentosa, pattern dystrophy, and fundus flavimaculatus in a single family with a deletion of codon 153 or 154 of the peripherin/RDS gene. Arch Ophthalmol 1993; 111: 1531–1542.
Wassle H . Parallel processing in the mammalian retina. Nat Rev 2004; 5: 1–11.
Petit-Jacques J, Völgyi B, Rudy B, Bloomfield S . Spontaneous oscillatory activity of starburst amacrine cells in the mouse retina. J Neurophysiol 2005; 94: 1770–1780.
Acknowledgements
This study was supported by grants from Hope for Vision, Foundation Fighting Blindness, Macula Vision Research Foundation, Chatlos Foundation, Research to Prevent Blindness, Ruth and Milton Steinbach Fund, and Alcon Research Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial interest: None
Rights and permissions
About this article
Cite this article
Aleman, T., Lam, B., Cideciyan, A. et al. Genetic heterogeneity in autosomal dominant retinitis pigmentosa with low-frequency damped electroretinographic wavelets. Eye 23, 230–233 (2009). https://doi.org/10.1038/eye.2008.264
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2008.264