Abstract
Periodontal disease is one of the most prevalent chronic disorders worldwide. It is accompanied by inflammation of the gingiva and destruction of periodontal tissues, leading to alveolar bone loss. Here, we focused on the role of adipokines, which are locally expressed by periodontal tissues, in the regulation of catabolic gene expression leading to periodontal inflammation. The expression of the nicotinamide phosphoribosyltransferase (NAMPT) adipokine was dramatically increased in inflamed human and mouse gingival tissues. NAMPT expression was also increased in lipopolysaccharide- and proinflammatory cytokine-stimulated primary cultured human gingival fibroblasts (GF). Adenovirus-mediated NAMPT (Ad-Nampt) overexpression upregulated the expression and activity of COX-2, MMP1 and MMP3 in human GF. The upregulation of IL-1β- or Ad-Nampt-induced catabolic factors was significantly abrogated by the intracellular NAMPT (iNAMPT) inhibitor, FK866 or by the sirtuin (SIRT) inhibitor, nicotinamide (NIC). Recombinant NAMPT protein or extracellular NAMPT (eNAMPT) inhibition using a blocking antibody did not alter NAMPT target gene expression levels. Moreover, intragingival Ad-Nampt injection mediated periodontitis-like phenotypes including alveolar bone loss in mice. SIRT2, a part of the SIRT family, was positively associated with NAMPT actions in human GF. Furthermore, in vivo inhibition of the NAMPT-NAD+-SIRT axis by NIC injection in mice ameliorated the periodontal inflammation and alveolar bone erosion caused by intragingival injection of Ad-Nampt. Our findings indicate that NAMPT is highly upregulated in human GF, while its enzymatic activity acts as a crucial mediator of periodontal inflammation and alveolar bone destruction via regulation of COX-2, MMP1, and MMP3 levels.
Similar content being viewed by others
Introduction
Periodontitis is a chronic inflammatory disease characterized by irreversible destruction of the periodontium and involves bacterial infection, periodontal inflammation, degradation of gum tissues and alveolar bone resorption. Periodontitis is mainly caused by pathogenic microorganisms, such as Porphyromonas gingivalis and Fusobacterium nucleatum.1 Periodontal pathogens may induce tissue damage by releasing endotoxins, such as lipopolysaccharides (LPS), which have profound effects on many cell types, including fibroblasts, osteoblasts, osteoclasts, and immune cells.2, 3 Periodontopathogens elicit inflammatory responses in the periodontium characterized by the local production of proinflammatory factors. These factors include tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-8, cyclooxygenase (COX)-2 (also known as prostaglandin G/H synthase (PTGS) 2) and matrix-degrading proteases, including matrix metalloproteinases (MMPs), all of which infiltrate into resident cells of the periodontium.4, 5, 6 Gingival connective tissue is predominantly composed of stromal cells, such as fibroblasts, and a collagen-rich extracellular matrix. Gingival fibroblasts (GF) are the most common cell type in gingival tissue and are exposed to periodontopathogens at an early stage of periodontal infection.7 Therefore, GF may have an important role in the transition from health to disease.
Recently, obesity, metabolic syndrome and diabetes mellitus have been significantly associated with periodontitis, suggesting that adipokines have a pathomechanistic role in theseassociations.8, 9, 10, 11 Serum levels of proinflammatory adipokines, such as nicotinamide phosphoribosyltransferase (NAMPT) (also called visfatin) and adiponectin, are increased in obesity and obesity-related diseases, and it has been speculated that such adipokines could enhance periodontal inflammation.12, 13, 14, 15, 16 Originally, adipokines were known to be secreted by adipocytes, but recently, they have also been shown to be locally produced by other cell types under pathophysiological conditions.17 Recent reports have demonstrated upregulated NAMPT expression in inflamed gingival crevicular fluid18 and gingival biopsy of periodontally diseased patients.19 Furthermore, NAMPT expression is upregulated in periodontal ligament (PDL) cells following infection of periodontopathogens,20, 21 and treatment with recombinant NAMPT stimulates increased expression of MMP1 and chemokine (C-C motif) ligand (CCL)2 in these cells.20 These observations demonstrate that gingival expression of NAMPT is increased at sites of periodontitis. However, the in vitro and in vivo functions of local NAMPT expression in the periodontium, including GF, as well as the mechanisms of NAMPT function in periodontitis need to be further clarified.
NAMPT functions in both intracellular (iNAMPT) and extracellular (eNAMPT) forms. iNAMPT exhibits enzymatic activity responsible for the salvaging pathways of nicotinamide adenine dinucleotide (NAD+) synthesis, while eNAMPT acts as an adipokine associated with inflammatory diseases.22, 23 A recent study, which focused on the function of eNAMPT as an adipokine, has suggested a role for adipokines in periodontal infection.17 Although this observation suggests a possible role of eNAMPT in periodontitis, the contribution of iNAMPT remains to be established. NAMPT enzymatic activity is an essential cofactor of sirtuin (SIRT) protein deacetylases. SIRT regulates gene expression by modulating chromatin function via direct deacetylation of histones and transcription factors.14, 22 In this study, we demonstrate that local gingival expression of NAMPT regulates the pathogenesis of periodontitis by modulating the expression of catabolic factors via SIRT activation in GF.
Materials and methods
Human gingival tissues
Human gingival tissues containing both epithelial and connective tissues were obtained from 16 patients (20–73 years; 40.80±18.80) during tooth extraction; eight healthy patients for non-inflamed gingiva and eight chronic periodontitis patients for inflamed gingiva. The Institutional Review Board at the Chonnam National University Dental Hospital (Gwangju, Republic of Korea) approved this study. Written informed consent was obtained from each study subject after all procedures had been fully explained. Gingival tissues were promptly maintained in liquid nitrogen and stored at −80 °C until further use.
Experimental animal model
Six-week-old female C57BL/6 mice were used as a periodontitis mouse model.24 Before bacterial inoculation, mice were administered antibiotics (2 mg ml−1 of sulfamethoxazole and 0.4 mg ml−1 of trimethoprim) in drinking water for three days, followed by 3 days without antibiotics. Mice were further inoculated with 1 × 1010 colony-forming units of P. gingivalis (ATCC, Manassas, VA, USA) in 1 ml phosphate-buffered saline with 2% carboxymethylcellulose (Sigma-Aldrich, St Louis, MO, USA). Inoculations were performed once a day for 3 days on the maxillary molar using phosphate-buffered saline as a vehicle. Mice were fostered for 40 days after the final inoculation of bacteria. Eight-week-old male C57BL/6 mice were intragingivally (IG) injected with adenovirus expressing Nampt (Ad-Nampt; 1 × 109 plaque-forming units in a total volume of 10 μl). Inoculations were performed once a day for 6 days at the region of the maxillary alveolar mucosa between the first and second molars using a Hamilton syringe (32 GA, 9.25 mm, 20°; Hamilton, Reno, NV, USA). IG injection of empty adenovirus (Ad-C) was used as a control. Mice were killed 18 days after the first IG injection for histological analyses. Where it was indicated, mice were co-injected intraperitoneally (IP) with 100 μl of nicotinamide (NIC) (300 mg kg−1 body weight; Sigma-Aldrich) once every 3 days. Mice were housed in specific pathogen-free barrier facilities and used in accordance with protocols approved by the Animal Care and Ethics Committees of Chonnam National University.
Cell culture and stimulation
Human GF were isolated from gingival papillary explants obtained from clinically healthy donors with no systemic and/or periodontal disease who were informed of the purpose of this study. Briefly, after dissection of gingival biopsies using dispase (Gibco BRL, Grand Island, NY, USA), the epithelial cell layer was microscopically dissected from the underlying connective tissue, and GF was extracted from the subepithelial tissue as previously described.25 Isolated primary human GF were treated with the indicated amounts of LPS derived from P. gingivalis (Invivogen, San Diego, CA, USA) in addition to recombinant human NAMPT protein (AdipoGen, San Diego, CA, USA), human IL-1β (GeneScript, Piscataway, NJ, USA) and human TNF-α (Merck-Millipore, Billerica, MA, USA). Primary cultured human GF were infected with empty (Ad-C), Nampt-expressing adenovirus (Ad-Nampt), or SIRT2-expressing adenovirus (Ad-SIRT2) for 2 h at the indicated multiplicity of infection and incubated for an additional 24 h. The inhibitors FK866 (Cayman, Ann Arbor, MI, USA), an iNAMPT inhibitor; anti-NAMPT antibody (Bio Vision, Milpitas, CA, USA), an eNAMPT inhibitor; NIC (Sigma-Aldrich), a total SIRT inhibitor; and EX-527 (Sigma-Aldrich), a SIRT1 inhibitor, were used at the indicated concentrations in the presence of IL-1β or Ad-Nampt. Dimethyl sulfoxide or phosphate-buffered saline was used as a vehicle.
Immunohistochemistry and immunofluorescence microscopy
Mouse maxillas were fixed in paraformaldehyde, decalcified, embedded in paraffin, sectioned (5 μm thickness) and processed for hematoxylin and eosin staining or immunohistochemistry. Sections were briefly incubated with the following primary antibodies: goat anti-COX2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-NAMPT (AdipoGen) and rabbit anti-MMP3 (Abcam, Cambridge, UK). To detect osteoclasts, a leukocyte acid phosphatase (tartrate-resistant acid phosphatase; TRAP) kit (Sigma-Aldrich) was used. For double immunofluorescence labeling of human GF, cells were cultured on glass coverslips and permeabilized with 0.1% Triton X-100. Cells were incubated for 1 h with primary antibodies followed by 1 h with an Alexa 488- or Alexa 594-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA). Images were acquired using a fluorescence microscope (Carl Zeiss, Cambridge, Jena, Germany) and were analyzed by counting positively stained cells using ImageJ software.
RNA isolation, PCR with reverse transcription (RT-PCR), quantitative real-time PCR (qRT-PCR) and western blotting
Total RNA was isolated from human gingival tissues and primary culture human GF using TRIzol reagent (Ambion, Carlsbad, CA, USA). Non-inflamed or inflamed human gingival tissues were homogenized in TRIzol reagent using a glass tissue grinder. RNA was reverse-transcribed, and the complementary DNA was amplified by PCR using Taq polymerase (GeneAll, Songpa, Seoul, Republic of Korea). Quantitative real-time PCR (qRT-PCR) was performed using an iCycler (Bio-Rad Laboratories, Hercules, CA, USA) and the SYBR premix Ex Taq (Takara Bio, Kyoto, Japan). All qRT-PCR was performed in duplicate, and the target gene amplification signal was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the same reaction. The relative levels of SIRT family (SIRT1–7) gene expression were analyzed using the comparative Ct (cycle threshold) method. The average Ct was calculated for SIRT1 to SIRT7 and GAPDH, as previously described.26 Primers and experimental conditions are shown in Supplementary Table 1. Western blot analysis was performed to detect the cellular and secreted levels of NAMPT, COX-2 and MMP3 in total cell lysates or in conditioned culture media using standard techniques. The following primary antibodies were used for western blotting: NAMPT (AdipoGen), MMP3 (Abcam), COX-2 (Cayman) and TUBULIN (Sigma-Aldrich).
Enzyme-linked immunosorbent assay (ELISA)
Primary cultured human GF were cultured under each experimental condition. For MMP and COX-2 activity assays, conditioned medium was harvested and centrifuged for 3 min at 842 g. The total MMP activity in collected supernatants was evaluated by using a SensoLyte 520 generic MMP assay kit (Anaspec, Fremont, CA, USA), and COX-2 activity was assayed using a PGE2 assay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Nuclear proteins were isolated for the SIRT deacetylase activity assay using the NE-PER Nuclear kit (Thermo Scientific, Rockford, IL, USA). Total SIRT deacetylase activity was measured using a colorimetric assay kit (Abcam) according to the manufacturer’s instructions. Absorbance was detected using an Epoch microplate reader (BioTek, Winooski, VT, USA) at 450 nm or FLx800 Fluorescence Reader (BioTek) at 520 nm.
Statistical analysis
The non-parametric Mann–Whitney U-test was used to analyze the ordinal grades (for example, cementoenamel junction to alveolar bone crest distance and bone surface to bone volume ratio). The data obtained with qRT-PCR and enzymatic activity assays were initially tested for conformation to normal distribution using the Shapiro–Wilk test and subsequently analyzed with Student’s t-test (pair-wise comparisons) or analysis of variance with post hoc tests (multi-comparison) where appropriate. The threshold for significance was set at the 0.05 level of probability (P<0.05).
Results
NAPMT is upregulated in inflamed human and mouse gingiva
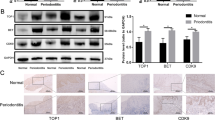
To investigate gingival expression of adipokines at sites of periodontitis, we examined the transcript levels of adipokines with previously detected expression in serum.8 We assessed the expression of adiponectin (ADIPOQ), apelin (APLN), leptin (LEP), resistin (RETN) and NAMPT. Of the examined adipokines, we observed that only NAMPT expression was highly upregulated in chronically inflamed gingival tissues obtained from human periodontitis patients compared with non-inflamed gingiva (Figures 1a and b). Upregulation of IL-6, IL-8, CCL5, PTGS2, MMP1 and MMP3 represents the severity of inflammation during periodontal disease (Figure 1a). To confirm the upregulation of NAMPT expression during periodontitis, we generated a P. gingivalis-infected periodontitis mouse model. Histomorphometric measurements of hematoxylin and eosin-stained tissue sections showed that the distance from the cementoenamel junction to alveolar bone crest was significantly greater in P. gingivalis-infected mice than in control mice (Figure 1c). Consistent with previous studies,19, 20 immunohistochemistry using an anti-NAMPT antibody showed NAMPT expression in gingival epithelial cells, GF, and PDL cells (Figure 1d). These data suggest that local gingival expression of NAMPT has a potential role in periodontal inflammation and subsequent alveolar bone erosion.
Increased expression of NAMPT in human and mouse inflamed gingiva. (a and b) Total RNA was extracted from whole gingival tissues of healthy (non-inflamed) or chronic periodontitis (inflamed) patients. mRNA levels of indicated adipokines were determined by RT-PCR (a). NAMPT expression was quantified by qRT-PCR (n=8) (b). (c and d) P. gingivalis was injected into the gingival margin of mouse maxillary molars for 3 days, and injection of empty adenovirus (Ad-C) was used as a control. H&E stained sections of intact periodontal tissues of control or P. gingivalis-infected mice and measurement of alveolar bone levels by comparing the distance from CEJ to ABC in μm (n=4) (c). Immunostaining of gingival tissue for NAMPT in control or P. gingivalis infected mice (d). GE, gingival epithelium; GCT, gingival connective tissue; PDL, periodontal ligament; AB, alveolar bone. Values are presented as the mean±s.e.m. (*P<0.05). Scale bar: 50 μm. ABC, alveolar bone crest; CEJ, cementoenamel junction; H&E, hematoxylin and eosin.
NAMPT expression is upregulated in pathogenic human GF
GF are the most abundant structural cells in periodontal tissue; they are exposed to pathogens at an early stage of periodontal infection and are critical in sustaining inflammation in periodontal disease.7 Therefore, we focused on NAMPT upregulation in human GF in inflamed gingiva. First, we examined expression patterns of adipokines following treatment with P. gingivalis-derived LPS (Pg LPS). We observed that Pg LPS induced increased expression of well-known periodontitis inflammatory factors, such as IL-6, IL-8, PTGS2, MMP1 and MMP3, that are correlated with the upregulation of NAMPT expression in a dose-dependent manner. The expression levels of other adipokines, including ADIPOQ, APLN, LEP and RETN, were very low or not detected (Figure 2a). These data are consistent with the results obtained using gingival tissues of periodontally diseased patients (Figure 1a). Moreover, proinflammatory cytokines IL-1β (Figure 2b) and TNF-α (Figure 2c), which are involved in periodontitis pathogenesis, dramatically increased NAMPT expression in a dose-dependent manner but did not increase the expression of other examined adipokines. The two different forms of NAMPT have distinct functions. iNAMPT acts as a regulator of cell metabolism and eNAMPT has a role in inflammation.22, 23 Following treatment with Pg LPS, IL-1β or TNF-α, pathogenic human GF produced both iNAMPT and eNAMPT (Figure 2d). In addition, IL-1β-induced NAMPT upregulation was abolished by inhibition of p38 kinase and NF-κB using SB203580 and SC514, respectively (Supplementary Figure 1).
NAMPT is upregulated in human GF during periodontal inflammation. (a) Primary cultures of human GF were left untreated or treated for 24 h with the indicated concentrations of LPS obtained from P. gingivalis. The mRNA levels of the indicated genes were detected by RT-PCR analysis. Relative NAMPT mRNA levels (compared to those of untreated cells) were quantified by qRT-PCR (n=5). (b and c) Human GF were treated with the indicated doses of cytokines IL-1β (b) and TNF-α (c) for 24 h. Transcript levels of the indicated genes were determined by RT-PCR and quantified by qRT-PCR analysis (n⩾5). (d) Western blot analysis of the extracellular (eNAMPT) and intracellular (iNAMPT) forms of NAMPT in human GF treated with the indicated concentrations of LPS, IL-1β and TNF-α. Values are presented as the mean±s.e.m. (*P<0.05, **P<0.01).
NAMPT regulates expression and activities of MMP1, MMP3 and COX2 in human GF
To explore the function of local NAMPT expression in gingiva during periodontitis pathogenesis, we overexpressed NAMPT. We examined the effects of NAMPT overexpression on the expression of catabolic factors involved in periodontal inflammation, including cytokines, chemokines, inflammatory mediators and matrix-degrading enzymes. Overexpression of NAMPT by Ad-Nampt infection in human GF led to a marked increase in the mRNA levels of PTGS2, MMP1 and MMP3 (Figure 3a). Additionally, increased protein levels of COX-2 and MMP3 were observed with immunoblotting (Figure 3b), and their increased enzymatic activities were determined by the overexpression of NAMPT (Figure 3c). Ad-Nampt-infected human GF expressed both eNAMPT and iNAMPT (Figure 3b), and cells with a high iNAMPT expression were strongly positive for target genes, such as COX-2 and MMP3, as shown in double-staining images (Figure 3d).
Ectopic expression of NAMPT regulates the expression of COX-2, MMP1 and MMP3 in human GF. Primary cultured human GF were left untreated (None) or were infected with 800 MOI of empty virus (Ad-C) or the indicated MOI of Nampt adenovirus (Ad-Nampt) for 24 h. (a) mRNA levels of the indicated genes were detected by RT-PCR (left) and quantified by qRT-PCR (n⩾5) (right). (b) Western blots of cellular NAMPT (iNAMPT), COX-2, MMP3 (Lysate), secreted NAMPT (eNAMPT) and MMP3 (Sup) in human GF infected with the indicated MOI of Ad-Nampt. (c) MMP activity and PGE2 release in human GF infected with Ad-C (800 MOI) or the indicated MOI of Ad-Nampt (n=5). (d) Immunofluorescence microscopy of NAMPT, COX-2 and MMP3 in human GF infected at an MOI of 800 with Ad-C or Ad-Nampt. DAPI was used for nuclear staining. Values are presented as the mean±s.e.m. (*P<0.05, **P<0.01). Scale bar, 50 μm. MOI, multiplicity of infection.
NAMPT enzymatic activity (iNAMPT) is necessary for NAMPT action
To further investigate the regulatory mechanism of gingival expression of NAMPT during periodontitis pathogenesis, we determined the inflammatory actions of iNAMPT and eNAMPT in human GF. Addition of human recombinant NAMPT protein (rNAMPT) to the culture media of GF, which essentially mimics eNAMPT action, did not induce the expression of PTGS2, MMP1 or MMP3 (Figure 4a). Treatment with a neutralizing antibody against NAMPT did not alter the IL-1β- (Figure 4b) or Ad-Nampt-induced upregulation of NAMPT target genes (Figure 4c). Treatment with FK866, a highly specific noncompetitive inhibitor of iNAMPT, in human GF significantly blocked the IL-1β- or Ad-Nampt-induced upregulation of PTGS2, MMP1 and MMP3 (Figures 4d and e). Moreover, FK866-mediated inhibition of iNAMPT enzymatic activity suppressed the actions of IL-1β (Figure 4f) and NAMPT overexpression by Ad-Nampt infection (Figure 4g) on the activation of MMPs and COX-2.
NAMPT enzyme activity is required for the upregulation of catabolic genes in human GF. (a) Expression levels of PTGS2, MMP1, and MMP3 as determined by RT-PCR in human GF treated with recombinant NAMPT (rNAMPT). (b and c) mRNA levels of catabolic factors determined by qRT-PCR in human GF treated with IL-1β (b), infected with Ad-Nampt (c) or exposed to the indicated concentrations of the NAMPT neutralizing antibody (n⩾5). (d and e) Human GF treated with IL-1β (d) or infected with Ad-Nampt (e) in the absence or presence of the indicated amounts of FK866 for 24 h. Expression levels of PTGS2, MMP1 and MMP3 were detected by qRT-PCR (n⩾5). (f and g) MMP activity and PGE2 release were measured in human GF treated with IL-1β (f) or infected with Ad-Nampt (g) in the presence of FK866 (n⩾4). Values are presented as the mean±s.e.m. (*P<0.05, **P<0.01).
NAMPT enzymatic activity is known to stimulate the synthesis of NAD+, an essential cofactor of SIRT protein deacetylases.22 Therefore, we next examined whether the effects of iNAMPT in human GF were due to the NAD+-SIRT axis. IL-1β treatment or Ad-Nampt infection increased total SIRT deacetylase activity in GF (Figures 5a and b). These increases were efficiently blocked by the inhibition of NAMPT using FK866 (Supplementary Figure 2), or by the inhibition of SIRT using NIC, a broad SIRT inhibitor (Figures 5a and b).27 As expected, inhibition of SIRT activity with NIC significantly blocked IL-1β-induced or Ad-Nampt-induced upregulation of PTGS2, MMP1 and MMP3 (Figures 5c and d) in addition to the activities of these target genes (Figures 5e and f). EX527, a SIRT1-specific inhibitor,28 had no effect on the expression of catabolic factors upregulated by IL-1β or NAMPT overexpression (Supplementary Figure 3). These results suggest that members of the SIRT family other than SIRT1 are associated with NAMPT-induced upregulation of COX-2, MMP1 and MMP3 in periodontal inflammation. Thus, we next determined which SIRT isoforms are involved in NAMPT regulation in GF. Although NAMPT overexpression increased SIRT activity, the mRNA levels of SIRT family members were not changed by overexpression of NAMPT (Figure 5g). Among the SIRT family members (SIRT1–7) examined, SIRT2 was found to be the most abundantly expressed in human GF (Figure 5h). Overexpression of SIRT2 by Ad-SIRT2 infection significantly increased the expression of the NAMPT target genes PTGS2, MMP1 and MMP3 (Figure 5i). Taken together, these results indicated that iNAMPT/SIRT activity, rather than eNAMPT, promotes the upregulation of PTGS2, MMP1 and MMP3 in pathogenic human GF.
The NAMPT-SIRT2 pathway mediates NAMPT-induced gene expression in human GF. (a–f) Human GF were untreated (None), treated with IL-1β (a, c and e), or infected with Ad-Nampt (b, d and f) in the absence or presence of NIC at the indicated concentrations. Total SIRT activity (a and b) and the expression levels of PTGS2, MMP1 and MMP3 (c and d) were determined (n=5). MMP activity and PGE2 production were measured in human GF treated with IL-1β or infected with Ad-Nampt in the presence of NIC (n⩾4) (e and f). (g) Primary cultured human GF were infected with Ad-C or Ad-Nampt at an MOI of 800. mRNA levels were determined by RT-PCR. (h) The relative amount of SIRT mRNA in untreated human GF was normalized to that of GAPDH mRNA. (i) Human GF were left untreated (None) or were infected with Ad-C (800 MOI) or Ad-SIRT2 at the indicated MOI. mRNA levels were determined by qRT-PCR (n⩾5). Values are presented as the mean±s.e.m. (*P<0.05, **P<0.001). MOI, multiplicity of infection.
Local gingival NAMPT is a regulator of periodontitis
NAMPT enzyme activity is required for COX-2 and MMP expression in pathogenic GF. Therefore, we examined the effects of iNAMPT on periodontitis in mice. Intragingival injection of Ad-Nampt induced alveolar bone loss, which was determined by volumetric measurements from μCT analysis (Figure 6a). The level of exposed root surface was found to be significantly higher in the maxilla (Figure 6b), while TRAP-positive osteoclasts were increased in the alveolar bone of Ad-Nampt-injected mice (Figure 6c). Consistent with these findings, upregulated expression of RANKL was detected in Ad-Nampt-infected human GF (Figure 6d). Co-injection of nicotinamide significantly ameliorated Ad-Nampt-induced periodontal bone destruction as determined by volumetric measurements from μCT analysis (Figure 6e) and histomorphometric measurements of hematoxylin and eosin-stained tissue sections displaying the cementoenamel junction to alveolar bone crest distance (Figure 6f). In addition, MMP3 upregulation and TRAP-positive osteoclast numbers were decreased by blockade of the NAMPT-SIRT axis (Figure 6g). Furthermore, increased RANKL transcript levels were reduced by SIRT inhibition in the presence of Ad-Nampt in human GF (Figure 6h). Collectively, these results demonstrate the essential role of NAMPT enzyme activity in periodontal inflammation and in stages subsequently following alveolar bone resorption.
Blockade of SIRT activity ameliorates NAMPT-mediated periodontal inflammation and alveolar bone loss. Mice were intragingivally injected with 1 × 109 PFU of empty virus (Ad-C) or Ad-Nampt (a–d), and co-injected intraperitoneally with NIC (e–h). After 18 days, mice were killed for further analysis. Periodontal inflammation and alveolar bone erosion were detected by volumetric measurements (BS to BV ratio) via μCT reconstruction (a and e) and histomorphometric analysis (distance from CEJ to ABC) using H&E stained sections (b and f) of the mice maxilla. NAMPT or MMP3 protein levels, and TRAP-positive osteoclasts in Ad-Nampt-infected gingival tissue with or without injection of NIC were examined by immunostaining (n=4) (c and g). Transcript level of RANKL was determined by qRT-PCR in GF infected with Ad-Nampt in the absence or presence of NIC (n=5) (d and h). Values are presented as the mean±s.e.m. (*P<0.05, **P<0.005). Scale bar: 50 μm. ABC, alveolar bone crest; BS, bone surface; BV, bone volume; CEJ, cementoenamel junction; H&E, hematoxylin and eosin.
Discussion
Periodontitis is caused by periodontopathogens in combination with other risk factors. Obesity and metabolic diseases enhance the risk for periodontitis and compromised periodontal healing. Adipokines are suggested to be pathomechanistic links between periodontitis and systemic inflammatory diseases.17 The pathogenesis of periodontitis primarily occurs through periodontal inflammation and alveolar bone resorption. Here, we demonstrate that iNAMPT serves as an essential catabolic regulator of periodontitis pathogenesis. iNAMPT upregulates COX-2, MMP1 and MMP3, all of which are critical effectors of periodontal inflammation, osteoclast activation and the likelihood of bone destruction around teeth.
Here, we focused on the upregulated expression of GF-derived NAMPT and demonstrated its possible roles in the modulation of periodontal inflammation and stages following alveolar bone loss. Adipokines are secreted mainly by adipose tissues and regulate insulin sensitivity, energy homeostasis and inflammation.12 A number of previous studies have suggested an association between periodontitis and systemic adipokines, such as NAMPT, along with a relationship between periodontitis, obesity and type 2 diabetes.29, 30 In periodontitis plasma levels, leptin31 and resistin32 are known to be increased, while gingival crevicular fluid leptin levels are known to be reduced.33 Recent studies have reported that increased NAMPT levels were detected in gingival crevicular fluid, serum18 and inflamed gingival biopsy 19 of human periodontally diseased patients. Plasma levels of NAMPT are also elevated in patients with obesity, metabolic syndrome, diabetes mellitus, cardiovascular diseases and insulin resistance.12 To investigate the possible involvement of systemic NAMPT during periodontal inflammation, we verified the effect of circulating NAMPT (eNAMPT) in human GF. Unexpectedly, we observed that eNAMPT was not sufficient in mediating the pathogenesis of periodontitis. Recombinant NAMPT protein and blocking antibody against NAMPT did not alter the NAMPT overexpression-mediated gene expression. However, whether eNAMPT functions in the regulation of other unexamined catabolic factors remains to be elucidated. Here, we examined the local expression of several adipokines in inflamed gingival tissue. With the exception of NAMPT, the local expression levels of the adipokines examined in inflamed gingival tissues and pathogenic human GF were extremely low. The expression of adiponectin, apelin, leptin and resistin was barely detected by qRT-PCR. Thus, we cautiously suggest that of the examined adipokines, NAMPT might be the most effective in locally regulating periodontal inflammation.
The role of NAMPT in inflammatory diseases has been widely investigated. NAMPT has been shown to stimulate a variety of cells to produce inflammatory mediators and proteases by using different intracellular pathways.29 However, NAMPT expression and regulatory mechanisms in periodontal cells have not been clearly defined yet. NAMPT expression was upregulated by F. nucleatum,20 P. gingivalis and IL-1β.21 Furthermore, NAMPT supplementation in PDL cells produced MMP1 and CCL2.21 Consistent with previous studies, our results demonstrate that NAMPT levels were markedly upregulated in inflamed gingival tissues from periodontitis patients and from a P. gingivalis-induced periodontitis mouse model. NAMPT upregulation in inflamed gingiva was verified by mimicking pathogenic conditions with LPS, IL-1β and TNF-α treatments. LPS, IL-1β and TNF-α are well-known proinflammatory cytokines involved in periodontitis in human GF. Pathogenic human GF produce significant amounts of NAMPT, cytokine (IL-6), chemokines (IL-8 and CCL5), inflammatory mediator (PTGS2) and matrix-degrading enzymes (MMP1 and MMP3), which are known to be expressed in pathogenic periodontium. Our gain-of-function studies using Ad-Nampt demonstrated that NAMPT upregulated various catabolic factors, including COX-2, MMP1 and MMP3 in human GF. Other types of adipokines are also known to upregulate catabolic gene expression, while leptin and proinflammatory cytokines may synergistically drive MMP1 and MMP3 production in human GF.34 Alveolar bone loss is a hallmark of periodontitis progression. The influx of inflammatory cells during periodontitis produces proinflammatory cytokines, such as IL-1, IL-6 and TNF-α, which have been implicated in the stimulation of osteoclastic resorption in periodontitis.35 These inflammatory cells also interact with periodontal stromal cells, such as osteoblasts, PDL cells and GF, resulting in the upregulation of RANKL, a mediator of osteoclast activation. Osteoblasts and GF stimulated by LPS and IL-1 induce RANKL expression.35 Here, we demonstrate that in human GF, RANKL expression is increased by Ad-Nampt-mediated NAMPT overexpression, while NIC-mediated inhibition of SIRT decreases the NAMPT-induced upregulation of RANKL. These results suggest that the NAMPT-SIRT axis plays a pivotal role in periodontal inflammation by regulating inflammatory mediators and MMPs, in addition to alveolar bone loss by regulating RANKL expression.
Our loss-of-function studies, by FK866 treatment in the presence of cytokines or Ad-Nampt, suggest that iNAMPT enzyme activity has an important role in the regulation of expression and activities of COX-2, MMP1 and MMP3. To date, the contribution of iNAMPT to periodontitis pathogenesis has not been established in vivo, although in vitro studies have suggested possible roles of eNAMPT in periodontitis.18, 19, 20, 21 Therefore, our study provides the first evidence for an in vivo function of iNAMPT in the pathogenesis of periodontitis. Intragingival injection of Ad-Nampt caused periodontitis, which was blocked by inhibition of the NAD+-SIRT axis. NAMPT enzymatic activity is known to stimulate the synthesis of NAD+,14, 22 an essential cofactor of SIRT protein deacetylases. NAMPT overexpression or IL-1β treatment increased NAD+-dependent SIRT activity in human GF, and SIRT activity was inhibited by NIC, a broad SIRT inhibitor.27 The mammalian SIRT family is composed of seven members, which are found in different subcellular locations, including the nucleus (SIRT6 and SIRT7), nucleus and cytosol (SIRT1 and SIRT2), and mitochondria (SIRT3–5). Among SIRT family members, SIRT1 is the best characterized in the periodontium. SIRT1 is thought to be involved in the differentiation of human PDL cells into osteoblast-like cells36 and in the remodeling of PDL in response to mechanical stress.37 However, EX527, a SIRT1-specific inhibitor,28 had no effect on NAMPT overexpression-induced upregulation of MMP1, MMP3 and PTGS2. We found that the endogenous expression level of SIRT2 was higher than that of other family members, although the expression of all seven SIRT proteins was not affected by Ad-Nampt infection. Involvement of SIRT2 in NAMPT-mediated PTGS2, MMP1 and MMP3 expression was verified by SIRT2 overexpression using Ad-SIRT2. Our results demonstrate that SIRT2 might be involved in NAMPT-induced gene expression in periodontal inflammation.
In summary, we demonstrated fundamental roles of the NAMPT-SIRT axis in human GF. NAMPT expression is increased in inflamed human and mouse gingival tissues during periodontitis. Furthermore, NAMPT has a pivotal role in the expression of catabolic factors (COX-2, MMP1, MMP3 and RANKL) in human GF, resulting in periodontal inflammation and alveolar bone loss. Effective treatment of periodontitis by various stimuli remains a significant medical need that is currently unachieved. Our findings suggest that future studies are warranted to examine the potential for targeting NAMPT and/or the NAMPT-SIRT axis as a therapeutic strategy in periodontitis.
References
Hajishengallis G . Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 2015; 15: 30–44.
Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B . Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun 1996; 64: 2371–2380.
Liu J, Wang S, Zhang P, Said-Al-Naief N, Michalek SM, Feng X . Molecular mechanism of the bifunctional role of lipopolysaccharide in osteoclastogenesis. J Biol Chem 2009; 284: 12512–12523.
Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H . Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther 2003; 100: 171–194.
Martinez FO, Sica A, Mantovani A, Locati M . Macrophage activation and polarization. Front Biosci J Virtual Libr 2008; 13: 453–461.
Naylor AJ, Filer A, Buckley CD . The role of stromal cells in the persistence of chronic inflammation. Clin Exp Immunol 2013; 171: 30–35.
Yucel-Lindberg T, Båge T . Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev Mol Med 2013; 15: e7.
Chaffee BW, Weston SJ . Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol 2010; 81: 1708–1724.
Nibali L, Tatarakis N, Needleman I, Tu Y-K, D’Aiuto F, Rizzo M et al. Clinical review: association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab 2013; 98: 913–920.
Chávarry NGM, Vettore MV, Sansone C, Sheiham A . The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Prev Dent 2009; 7: 107–127.
Preshaw PM, Foster N, Taylor JJ . Cross-susceptibility between periodontal disease and type 2 diabetes mellitus: an immunobiological perspective. Periodontol 2000 2007; 45: 138–157.
Chang Y-H, Chang D-M, Lin K-C, Shin S-J, Lee Y-J . Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev 2011; 27: 515–527.
Taşkesen D, Kirel B, Us T . Serum visfatin levels, adiposity and glucose metabolism in obese adolescents. J Clin Res Pediatr Endocrinol 2012; 4: 76–81.
Zhang LQ, Heruth DP, Ye SQ . Nicotinamide phosphoribosyltransferase in human diseases. J Bioanal Biomed 2011; 3: 13–25.
Lakkis D, Bissada NF, Saber A, Khaitan L, Palomo L, Narendran S et al. Response to periodontal therapy in patients who had weight loss after bariatric surgery and obese counterparts: a pilot study. J Periodontol 2012; 83: 684–689.
Tilg H, Moschen AR . Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin Sci (Lond) 2008; 114: 275–288.
Deschner J, Eick S, Damanaki A, Nokhbehsaim M . The role of adipokines in periodontal infection and healing. Mol Oral Microbiol 2014; 29: 258–269.
Pradeep AR, Raghavendra NM, Prasad MVR, Kathariya R, Patel SP, Sharma A . Gingival crevicular fluid and serum visfatin concentration: their relationship in periodontal health and disease. J Periodontol 2011; 82: 1314–1319.
Damanaki A, Nokhbehsaim M, Eick S, Götz W, Winter J, Wahl G et al. Regulation of NAMPT in human gingival fibroblasts and biopsies. Mediators Inflamm 2014; 2014: 912821.
Nogueira AVB, Nokhbehsaim M, Eick S, Bourauel C, Jäger A, Jepsen S et al. Regulation of visfatin by microbial and biomechanical signals in PDL cells. Clin Oral Investig 2014; 18: 171–178.
Nokhbehsaim M, Eick S, Nogueira AVB, Hoffmann P, Herms S, Fröhlich H et al. Stimulation of MMP-1 and CCL2 by NAMPT in PDL cells. Mediators Inflamm 2013; 2013: 437123.
Yang S, Ryu JH, Oh H, Jeon J, Kwak JS, Kim JH et al. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2α, is an essential catabolic regulator of osteoarthritis. Ann Rheum Dis 2015; 74: 595–602.
Imai S-I . Nicotinamide phosphoribosyltransferase (Nampt): a link between NAD biology, metabolism, and diseases. Curr Pharm Des 2009; 15: 20–28.
Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC . CD4+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun 1999; 67: 2804–2809.
Moon JS, Cheong NR, Yang SY, Kim IS, Chung HJ, Jeong YW et al. Lipopolysaccharide-induced indoleamine 2,3-dioxygenase expression in the periodontal ligament. J Periodontal Res 2013; 48: 733–739.
Oh H, Kwak JS, Yang S, Gong MK, Kim JH, Rhee J et al. Reciprocal regulation by hypoxia-inducible factor-2α and the NAMPT-NAD(+-SIRT axis in articular chondrocytes is involved in osteoarthritis. Osteoarthritis Cartilage 2015; 23: 2288–2296.
Avalos JL, Bever KM, Wolberger C . Mechanism of sirtuin inhibition by nicotinamide: altering the NAD+ cosubstrate specificity of a Sir2 enzyme. Mol Cell 2005; 17: 855–868.
Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol 2006; 26: 28–38.
Dahl TB, Holm S, Aukrust P, Halvorsen B . Visfatin/NAMPT: a multifaceted molecule with diverse roles in physiology and pathophysiology. Annu Rev Nutr 2012; 32: 229–243.
Zimmermann GS, Bastos MF, Dias Gonçalves TE, Chambrone L, Duarte PM . Local and circulating levels of adipocytokines in obese and normal weight individuals with chronic periodontitis. J Periodontol 2013; 84: 624–633.
Shimada Y, Komatsu Y, Ikezawa-Suzuki I, Tai H, Sugita N, Yoshie H . The effect of periodontal treatment on serum leptin, interleukin-6, and C-reactive protein. J Periodontol 2010; 81: 1118–1123.
Patel SP, Raju PA . Resistin in serum and gingival crevicular fluid as a marker of periodontal inflammation and its correlation with single-nucleotide polymorphism in human resistin gene at -420. Contemp Clin Dent 2013; 4: 192–197.
Karthikeyan BV, Pradeep AR . Gingival crevicular fluid and serum leptin: their relationship to periodontal health and disease. J Clin Periodontol 2007; 34: 467–472.
Williams RC, Skelton AJ, Todryk SM, Rowan AD, Preshaw PM, Taylor JJ . Leptin and pro-inflammatory stimuli synergistically upregulate MMP-1 and MMP-3 secretion in human gingival fibroblasts. PloS ONE 2016; 11: e0148024.
Hienz SA, Paliwal S, Ivanovski S . Mechanisms of bone resorption in periodontitis. J Immunol Res 2015; 2015: 615486.
Lee Y-M, Shin S-I, Shin K-S, Lee Y-R, Park B-H, Kim E-C . The role of sirtuin 1 in osteoblastic differentiation in human periodontal ligament cells. J Periodontal Res 2011; 46: 712–721.
Lee S-I, Park K-H, Kim S-J, Kang Y-G, Lee Y-M, Kim E-C . Mechanical stress-activated immune response genes via Sirtuin 1 expression in human periodontal ligament cells. Clin Exp Immunol 2012; 168: 113–124.
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (2011-0030121 and NRF-2015R1D1A1A01057870) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) as well as funded by the Ministry of Health & Welfare (HI16C0287 and HI14C3484) and Chonnam National University Hospital Biomedical Research Institute (CRI16077-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Park, K., Kim, DK., Huh, Y. et al. NAMPT enzyme activity regulates catabolic gene expression in gingival fibroblasts during periodontitis. Exp Mol Med 49, e368 (2017). https://doi.org/10.1038/emm.2017.116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2017.116