Abstract
Autoimmune diseases (AIDs), a heterogeneous group of immune-mediated disorders, are a major and growing health problem. Although AIDs are currently treated primarily with anti-inflammatory and immunosuppressive drugs, the use of stem cell transplantation in patients with AIDs is becoming increasingly common. However, stem cell transplantation therapy has limitations, including a shortage of available stem cells and immune rejection of cells from nonautologous sources. Induced pluripotent stem cell (iPSC) technology, which allows the generation of patient-specific pluripotent stem cells, could offer an alternative source for clinical applications of stem cell therapies in AID patients. We used nonintegrating oriP/EBNA-1-based episomal vectors to reprogram dermal fibroblasts from patients with AIDs such as ankylosing spondylitis (AS), Sjögren’s syndrome (SS) and systemic lupus erythematosus (SLE). The pluripotency and multilineage differentiation capacity of each patient-specific iPSC line was validated. The safety of these iPSCs for use in stem cell transplantation is indicated by the fact that all AID-specific iPSCs are integrated transgene free. Finally, all AID-specific iPSCs derived in this study could be differentiated into cells of hematopoietic and mesenchymal lineages in vitro as shown by flow cytometric analysis and induction of terminal differentiation potential. Our results demonstrate the successful generation of integration-free iPSCs from patients with AS, SS and SLE. These findings support the possibility of using iPSC technology in autologous and allogeneic cell replacement therapy for various AIDs, including AS, SS and SLE.
Similar content being viewed by others
Introduction
Autoimmune diseases (AIDs) are caused by immunological imbalance and the loss of tolerance of self-antigens, both of which cause the immune system to destroy self-tissues. AIDs comprise >80 different diseases and affect ∼100 million people worldwide.1 Autoimmunity can damage all tissues and cells in the body. AIDs can be classified into two major categories.2 Some AIDs, such as type 1 diabetes, which attacks the pancreas, and autoimmune hemolytic anemia, which targets erythrocytes, are organ specific, whereas other AIDs, such as systemic lupus erythematosus (SLE), rheumatoid arthritis, ankylosing spondylitis (AS), inflammatory bowel disease and Sjögren’s syndrome (SS), are systemic and affect multiple organs. For most patients with AIDs, conventional therapy with anti-inflammatory and immunosuppressive agents provides effective treatment. Nonetheless, some patients are resistant to these drugs and may require stem cell-based cell replacement therapies, such as hematopoietic stem cell transplantation or mesenchymal stem cell transplantation.3 Stem cell-based cell replacement has been used as an alternative treatment for many AIDs, including multiple sclerosis, systemic sclerosis, rheumatoid arthritis, SLE, Crohn’s disease, type 1 diabetes, AS and SS.4, 5 However, the application of stem cell transplantation is limited by the shortage of stem cells and by the potential for immune rejection of cells from nonautologous sources.6, 7
Induced pluripotent stem cells (iPSCs), which can be obtained from various cell types of an individual, provide valuable human cell resources for disease modeling, drug discovery and regenerative medicine.8 iPSCs can be generated from a patient’s own cells by the forced expression of selected transcription factors and share similar properties with embryonic stem cells (ESCs), including the capacity for indefinite proliferation (self-renewal) and multilineage differentiation potential (pluripotency).9, 10 Patient-specific iPSCs have emerged as promising candidates for cell replacement therapy because the use of such cells avoids the problems associated with immunological rejection and ethical issues and provides a limitless source of cells for translational application.11 Moreover, patient-specific iPSCs and their differentiated derivatives can provide a unique platform in which to model a disease in vitro and to screen the effectiveness of drugs in individual patients. However, the current reprogramming technique to generate iPSCs still needs to be improved, including the viral delivery, the integration of transgene into the genome and low reprogramming efficiency.12
In this study, we successfully generated ‘footprint-free’ AID-specific iPSCs from patients with AS, SS and SLE using nonintegrating episomal vectors. The iPSCs derived through this method expressed ESC markers and showed potential for differentiation into all three germ layers both in vitro and in vivo. These AID-specific iPSCs could be differentiated into hematopoietic and mesenchymal lineage cells. These findings support the feasibility of using iPSC technology in cell replacement therapy for various AIDs. To our knowledge, this is the first report to describe the generation of clinically relevant iPSCs from patients with AS, SS and SLE and to demonstrate their potential as valuable sources of patient-derived autologous cells.
Materials and methods
Patients and samples
Patient with AS fulfilled the modified New York Criteria.13 Patient with SS fulfilled the European criteria.14 Patient with SLE fulfilled the American College of Rheumatology criteria.15 Dermal fibroblasts were obtained by biopsy from patients with AS, SS and SLE. This study was officially approved by the Korea National Institute for Bioethics Policy (Public Institutional Review Board, Seoul, Korea, IRB No. P01-201404-BS-05), and written informed consent was obtained in accordance with the principles of the Declaration of Helsinki.
Human iPSC generation and culture
To generate integration-free iPSC lines, 1 × 106 fibroblasts derived from patients were electroporated with Episomal iPSC Reprogramming Vectors (Cat. No. A14703, Invitrogen, Carlsbad, CA, USA) using the Neon Transfection System (Cat. No. MPK10096, Invitrogen) at a pulse voltage of 1650 V, a pulse width of 10 ms and a pulse number of 3 according to the manufacturer’s instructions. The electroporated cells were evenly plated on two sets of 35 mm dishes. At 5 days after electroporation, the fibroblasts were seeded at 1 × 105 onto Matrigel (BD Biosciences, San Diego, CA, USA)-coated 6-well plate. The cells were fed daily with mTeSR1 medium (Stem Cell Technologies, Vancouver, BC, Canada) or with human iPSC medium consisting of Dulbecco’s modified Eagle’s medium/F12 medium (Invitrogen) supplemented with 20% Knockout SR (Invitrogen), 1% nonessential amino acids (NEAA, Invitrogen), 1 mM L-glutamine (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma, St Louis, MO, USA) and 10 ng ml−1 basic fibroblast growth factor (Invitrogen). To increase reprogramming efficiency, nicotinamide was added at 1 mM. After 14 or 21 days, human ESC-like iPSC colonies were picked, subsequently passaged and expanded for further characterization. Patient-specific human iPSC lines were grown on Matrigel in mTeSR1 or MEF-conditioned medium as described previously.16, 17 Patient-derived fibroblasts were maintained in DMEM containing 10% fetal bovine serum (Invitrogen), 1% NEAA, 1 mM L-glutamine and 0.1 mM β-mercaptoethanol.
Alkaline phosphatase and immunofluorescence staining
For alkaline phosphatase staining, iPSCs were fixed in a citrate–acetone–formaldehyde solution and then stained with alkaline phosphatase staining solution (Naphthol/fast red violet, Sigma). Cell images were captured using an Olympus microscope (IX51, Olympus, Tokyo, Japan).
For immunofluorescence staining, cells were fixed in 4% formaldehyde and then permeabilized with phosphate-buffered saline containing 0.1% Triton X-100. After blocking with 3% bovine serum albumin, cells were incubated with the following primary antibodies: OCT4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA, sc-9081, 1:200), NANOG (Santa Cruz Biotechnology, sc-33759, 1:100), TRA-1-81 (Chemicon, Santa Cruz, CA, USA, MAB4381, 1:100), TRA-1-60 (Chemicon, MAB4360, 1:100), SSEA3 (R&D Systems, Minneapolis, MN, USA, MAB1434, 1:100), SSEA4 (R&D Systems, MAB1435, 1:100), DESMIN (Chemicon, AB907, 1:50), α-smooth muscle actin (Sigma-Aldrich, Carlsbad, CA, USA, A5228, 1:400), FOXA2 (Abcam, Cambridge, MA, USA, ab40874, 1:500), SOX17 (R&D Systems, MAB1924, 1:100), TUJ1 (Covance, Munich, Germany, PRB-435P, 1:500) and NESTIN (Chemicon MAB5326, 1:100). DAPI (4',6-diamidino-2-phenylindole) was used for nuclear counterstaining. Chamber slides were observed with an Axiovert 200M microscope (Carl Zeiss, Gottingen, Germany) or an Olympus microscope (Olympus).
Quantitative real-time reverse transcription-PCR
Total RNA was extracted from cells using an RNeasy Kit (Qiagen, Valencia, CA, USA) and reverse-transcribed using a Superscript III cDNA synthesis kit (Invitrogen). Quantitative real-time reverse transcription-PCR (qRT-PCR) was performed in an ABI PRISM 7500 Fast Real-time PCR system (Applied Biosystems, Foster City, CA, USA). The primers used in this study have been previously described.18 All experiments were run in triplicate, and a CT value for each target gene was determined using the software provided by the manufacturer.
Episomal copy number detection
Human iPSC pellets were lysed with 200 μl of lysis solution containing 1 × Taq buffer (Takara, Kyoto, Japan) and proteinase K at 55 °C for 3 h. The lysates were used for qRT-PCR analysis as previously described.19 The pCXLE-hFbx15-cont2 plasmid was used to generate a standard curve. The copy number of EBNA-1 (Epstein–Barr nuclear antigen 1) and FBXO15 in each sample was estimated from the CT values observed in six reactions.
Short tandem repeat analysis
Short tandem repeat analysis to analyze the origins of patient-specific iPSC lines was conducted by HumanPass (Seoul, Korea).
In vitro differentiation based on the formation of embryoid bodies (EBs)
For spontaneous differentiation through EB formation, human iPSCs were dissociated by treatment with 1 mg ml−1 collagenase IV and transferred to Petri dishes in EB medium consisting of knockout DMEM supplemented with 10% KSR, 1% NEAA, 0.1 mM β-mercaptoethanol and 1 mM L-glutamine. After 5 days in suspension culture, EBs were transferred to gelatin-coated plates and cultured for an additional 10 days.
Teratoma injection
Undifferentiated iPSCs (1 × 106) were mixed with Matrigel and injected subcutaneously into the dorsolateral area of specific pathogen-free/viral antibody-free immunodeficient mice (Orient Bio, Seoul, Korea). The mice were maintained under specific pathogen free (SPF) conditions and fed a sterilized pelleted diet and water. At 8 weeks after injection, the tumor tissues were dissected and fixed in 4% paraformaldehyde. Paraffin-embedded tissues were sectioned and then stained with hematoxylin and eosin.
Differentiation into HSCs
Patient-specific iPSCs were differentiated into hematopoietic stem cells (HSCs) under defined, serum-free and feeder-free conditions as previously reported.20 Briefly, TrypLE (Invitrogen)-dissociated iPSCs were seeded onto 6-well plates that were precoated with 3 mg cm−2 human plasma fibronectin (Invitrogen) in mTeSR1 supplemented with an inhibitor of Rho-associated kinase (H1152, Sigma). After 1 day, mTeSR1 was replaced with a hematopoietic commitment medium containing Iscove’s modified Dulbecco’s medium (Invitrogen) supplemented with 1 × HIT (Stem Cell Technologies), 450 μM monothioglycerol (Sigma), 50 ng ml−1 recombinant human BMP4 (R&D Systems), 50 ng ml−1 recombinant human vascular endothelial growth factor (Invitrogen), 0.1 mM NEAA (Invitrogen) and 2 mM L-glutamine (Invitrogen). After 6 days, the medium was changed to a hematopoietic maturation medium consisting of Iscove’s modified Dulbecco’s medium (Invitrogen) supplemented with 5 U ml−1 heparin (Sigma), 25 ng ml−1 thrombopoietin (Invitrogen), 25 ng ml−1 human recombinant stem cell factor (Invitrogen), 25 ng ml−1 human recombinant FLT3L (Peprotech, Rocky Hill, NJ, USA), 10 ng ml−1 interleukin-3 (Invitrogen) and 10 ng ml−1 interleukin-6 (Invitrogen). The cells were incubated under hypoxic conditions (5% O2 balanced with nitrogen).
Differentiation into MSCs
Human iPSCs were transferred to Matrigel-coated dishes and cultured in mTESR1 medium for 5 days. The iPSCs were differentiated to mesenchymal stem cells (MSCs) by adding MSC differentiation medium containing minimum essential medium-α supplemented with 10% fetal bovine serum, 1% NEAA, 1% GlutaMAX and 5 ng ml−1 basic fibroblast growth factor and passaged every 7–10 days.
Flow cytometry
Cells were dissociated using 0.25% trypsin/EDTA and suspended at 1 × 105 cells in 100 μl staining buffer (1% fetal bovine serum+1 mM EDTA in Dulbecco’s phosphate-buffered saline). The cells were labeled with antibodies against CD43-PE, CD45-APC, CD34-APC, CD73-PE and CD90-PE (Miltenyl Biotec, Cologne, Germany) at 4 °C for 30 min, washed twice with staining buffer and then analyzed on a BD Accuri C6 flow cytometer (BD Biosciences, Bedford, MA, USA) according to the manufacturer’s instructions. The data were analyzed using FlowJo V10 software (FLOWJO, Ashland, OR, USA).
CFU assay for iPSC-derived HSCs
The colony-forming unit (CFU) assay was performed using a methylcellulose-based serum-free medium (MethoCult Optimum, Cat. No. 04034, Stem Cell Technologies) according to the manufacturer’s instructions. CD45+ HSCs purified by MACS (CD45 MicroBeads, human, Miltenyi Biotech, Auburn, CA, USA) were cultured for 3 weeks. CFU numbers were scored manually using an inverted microscope.
Differentiation into adipocytes, chondrocytes and osteocytes
Mesenchymal differentiation of human iPSCs was induced using specific osteogenic, chondrogenic and adipogenic differentiation media. For adipocyte differentiation, cells were cultured in DMEM containing 10% Knockout SR, 0.1 mM β-mercaptoethanol, 2 mM glutamine, 1% NEAA, 100 nM retinoic acid, 0.5 mM 3-isobutyl-L-methylxanthine (Sigma), 1 μg ml−1 insulin (Sigma) and 0.25 μM dexamethasone (Sigma) for 3 weeks. For differentiation into osteocytes, cells were cultured in DMEM containing 0.1 mM L-ascorbic acid (Sigma), 10 mM dexamethasone and 1 M indomethacin for 3 weeks. For chondrocyte differentiation, cells were cultured in DMEM containing 1% Knockout SR, 2 mM L-glutamine, 1% NEAA, 40 μg ml−1 L-proline (Sigma), 50 μg ml−1 ascorbic acid 2-phosphate (Sigma), 1% sodium pyruvate, 1% ITS (6.25 mg ml−1 insulin, 6.25 mg ml−1 transferrin and 6.25 ng ml−1 selenium), 1.25 mg ml−1 bovine serum albumin, 5.35 mg ml−1 linoleic acid (BD Bioscience, Franklin Lakes, NJ, USA), 0.1 μM dexamethasone and 100 ng ml−1 BMP2 for 3 weeks.
Results
Generation of integration-free iPSCs from patients with AIDs
We obtained dermal fibroblasts from biopsy samples from patients with AS, SS and SLE. To derive transgene integration-free iPSCs, each patient-derived fibroblast was reprogrammed into iPSCs using oriP/EBNA-1-based episomal vectors encoding OCT4, SOX2, KLF4, L-MYC, LIN28 and shRNA-p53 (Figure 1a).19 However, the reprogramming efficiency was very low (~0.0003%) as previously described.19, 21 Thus, we used nicotinamide, which we showed in a previous study21 to be a reprogramming booster for efficient human iPSC generation, during the reprogramming process. Supplementation of the culture medium with 1 mM nicotinamide enhanced the reprogramming efficiency more than 30-fold in AS-fibroblasts (~0.009±0.002%), 131-fold in SS-fibroblasts (~0.039±0.013%) and 220-fold (~0.044±0.025%) in SLE-fibroblasts. AID-specific iPSC colonies were picked at 14–21 days after transfection with episomal vectors.
Establishment of autoimmune disease (AID)-specific induced pluripotent stem cells (iPSCs). (a) Schematic diagram of the reprogramming protocol. Dermal fibroblasts derived from patients with ankylosing spondylitis (AS), Sjögren syndrome (SS) and systemic lupus erythematosus (SLE) were reprogrammed into iPSCs using nonintegrating episomal vectors. (b) The morphology of representative patient-specific fibroblasts and iPSC colonies. All iPSC colonies expressed alkaline phosphatase (ALP). Scale bar, 100 μm. (c) Immunofluorescence analysis of common human embryonic stem cell (hESC) markers (OCT4, NANOG, SSEA-4 and TRA-1-60) in patient-specific iPSCs derived from patients with AS, SS and SLE. Scale bar, 100 μm. (d) Quantitative real-time reverse transcription-PCR (qRT-PCR) analysis of gene expression for OCT4 and NANOG was performed in fibroblasts and in iPSCs derived from patients with AS, SS and SLE. Fold changes in expression levels are relative to donor fibroblasts. The data are presented as mean (s.e.m.) (n=3).
All AID-specific iPSC lines, including AS-iPSCs, SS-iPSCs and SLE-iPSCs, retained undifferentiated human ESC-like morphology, with distinct edges and a high nucleus-to-cytoplasm ratio, and exhibited alkaline phosphatase activity (Figure 1b). Immunofluorescence analysis (Figure 1c) showed that AS-iPSCs, SS-iPSCs and SLE-iPSCs expressed ESC markers, including OCT4 and NANOG, and cell surface markers, including TRA-1-81, TRA-1-60, SSEA3 and SSEA4. The endogenous expression of OCT4 and NANOG in all AID-specific iPSC lines was further confirmed by qRT-PCR analysis using fibroblasts from each patient as a control (Figure 1d).
Characterization of AID-specific iPSCs generated using the episomal reprogramming method
Next, to evaluate the iPSCs as clinically relevant cell sources, we estimated the copy numbers of the episomal vectors in the AID-specific iPSC lines. At 5 days after transfection, we detected ∼75, 171 and 211 copies of the episomal vector per AS-fibroblast, SS-fibroblast and SLE-fibroblast, respectively. In contrast, all established AID-specific iPSC lines displayed no transgene genomic integration after 47 passages, as determined by qRT-PCR using primer pairs specific for the EBNA-1 sequence and the endogenous FBXO15 locus (Figure 2a). These results demonstrate that all AID-specific iPSC lines were completely free of the episomal vectors and that the episomal vector-mediated reprogramming did not cause any detectable alterations in the genome. The genetic identity of each AS-iPSC, SS-iPSC and SLE-iPSC to the donor patient’s fibroblasts was confirmed by short tandem repeat analysis; the results showed that no contamination had occurred during the reprogramming and maintenance of the iPSCs (Figure 2b).
Generation of transgene-free induced pluripotent stem cells (iPSCs) from patients with autoimmune diseases (AIDs). (a) Copy numbers of episomal vectors in patient-specific iPSCs derived from patients with ankylosing spondylitis (AS), Sjögren syndrome (SS) and systemic lupus erythematosus (SLE). The passage number of each iPSC line is shown in parentheses. Each patient-derived fibroblast was analyzed 5 days after electroporation as a positive control. (b) Short tandem repeat (STR) profiles of patient-specific iPSCs. The shaded areas indicate the donor cells for each iPSC clone.
Validation of the pluripotency of AID-specific iPSCs
The pluripotency of AID-specific iPSC lines was further demonstrated both in vitro and in vivo. Spontaneous differentiation of the cells via EB formation permitted determination of whether the derived AID-specific iPSC lines differentiate into the various cell types of the three germ layers in vitro. After 5 days in suspension culture, EBs were allowed to attach to gelatin-coated dishes for 10 days under differentiation conditions. Immunofluorescence analysis using markers specific for the individual embryonic germ layers confirmed that AS-iPSCs, SS-iPSCs and SLE-iPSCs differentiated into representative derivatives of endoderm (SOX17 and FOXA2), mesoderm (DESMIN and α-smooth muscle actin) and ectoderm (TUJ1 and NESTIN) (Figure 3ai–iii).
In vitro and in vivo differentiation potential of autoimmune disease (AID)-specific induced pluripotent stem cells (iPSCs). (a) In vitro differentiation of patient-specific iPSCs derived from patients with (ai) ankylosing spondylitis (AS), (aii) Sjögren syndrome (SS) and (aiii) systemic lupus erythematosus (SLE). Immunofluorescence analyses of AID-specific iPSCs for endodermal markers SOX17 and FOXA2, mesodermal markers DESMIN and α-smooth muscle actin (α-SMA) and ectodermal markers TUJ1 and NESTIN were performed. Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI; blue). Scale bar, 100 μm. (b) In vivo differentiation assay using teratoma formation. Hematoxylin and eosin-stained sections from teratomas generated from patient-specific iPSCs derived from patients with (bi) AS, (bii) SS and (biii) SLE (original magnification, × 200) are shown. The differentiation of the teratomas into multiple derivatives of the three germ layers is shown.
To confirm the in vivo pluripotency of the AID-specific iPSC lines, we also subcutaneously transplanted the cultured AID-specific iPSC lines into the dorsal flanks of immunodeficient mice in a teratoma assay. Histological analysis demonstrated that AS-iPSCs, SS-iPSCs and SLE-iPSCs developed into primitive tissues representing all three germ layers, including ectoderm (neural rosette and epidermis containing melanocytes), mesoderm (cartilage, adipocyte, myxoid, bone and blood vessel) and endoderm (gut-like epithelium) (Figure 3bi–iii). These results demonstrate that the established and cultured AID-specific iPSC lines retain pluripotency in vitro and in vivo.
Hematopoietic differentiation potential of AID-specific iPSCs
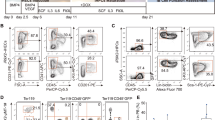
To examine the hematopoietic differentiation potential of AID-specific iPSCs, we differentiated AS-iPSCs, SS-iPSCs and SLE-iPSCs into HSCs using a defined, feeder-free, serum-free system, as reported previously (Figure 4a).20 On day 10 of the differentiation process, each patient-specific iPSC-derived HSC expressed the early hematopoietic marker CD34 (26.3%, 62.7% and 60.0% in AS-HSCs, SS-HSCs and SLE-HSCs, respectively), the committed hematopoietic progenitor marker CD43 (21.4%, 45.3% and 47.7%, respectively) and the human pan-hematopoietic marker CD45 (58.8%, 82.3% and 76.2%, respectively) (Figure 4b). In addition, all iPSC-derived HSCs displayed multilineage differentiation capacity, as shown by their ability to differentiate into CFU-E (erythroid), CFU-G (granulocyte), CFU-M (macrophage) and CFU-GM (granulocyte, macrophage) cells (Figure 4c). An analysis of the numbers of CFUs of each of the lineage-committed cells per 2 × 104 cells showed that each iPSC-derived AS-HSC, SS-HSC and SLE-HSC could be efficiently differentiated into hematopoietic lineage cells, although SLE-HSCs showed a somewhat lower differentiation efficiency (Figure 4d). These results suggest that our AID patient-specific iPSCs have the potential to provide a viable cell source for hematopoietic stem cell transplantation.
Differentiation of autoimmune disease (AID)-specific induced pluripotent stem cells (iPSCs) into various hematopoietic lineages. (a) A schematic diagram of the protocol used to obtain multipotent hematopoietic stem cells (HSCs) from patient-specific iPSCs obtained from patients with ankylosing spondylitis (AS), Sjögren syndrome (SS) and systemic lupus erythematosus (SLE). (b) Fluorescence-activated cell sorting (FACS) analysis of HSCs derived from patients with specific AIDs. After 14 days of differentiation, cells were analyzed by flow cytometry for the expression of CD34, CD43 and CD45. The data are presented as mean (s.e.m.) of three individual experiments. (c) Colony-forming unit (CFU) assay of AID-specific iPSC-derived HSCs. The left panel shows the morphology of the CFUs, and the right panel shows the number of CFUs per 2 × 104 cells (d). CFU-E, CFU-erythroid; CFU-G, CFU-granulocyte; CFU-M, CFU-macrophage; CFU-GM, CFU-granulocyte and macrophage. The right panel shows the average CFU values per 2 × 104 cells at day 21.
Mesenchymal differentiation potential of AID-specific iPSCs
We next examined whether AID-specific iPSCs could be differentiated into MSCs and whether iPSC-derived MSCs can differentiate into mesenchymal lineages using a standard differentiation protocol (Figure 5a). To determine whether the morphologically fibroblastic-shaped cells differentiated from AID-specific iPSCs were MSCs, the cells were analyzed by flow cytometry. The results showed that the MSC surface markers CD90 and CD73 were highly expressed, at levels ranging from 86.8 to 99.8%, in AID-specific iPSC-derived MSCs (Figure 5b). To confirm the mesenchymal lineage differentiation potential of the AID-specific iPSCs, we attempted to differentiate AS-iPSCs, SS-iPSCs and SLE-iPSCs into osteocytes, chondrocytes and adipocytes by incubating the cells in lineage-specific medium. Histological analysis by Oil red O staining for intracellular lipid droplets, Alcian blue staining for proteoglycans and Alizarin red staining for calcium deposits demonstrated that the AID-specific iPSCs differentiated into adipocyte, chondrocyte and osteoblast lineages (Figure 5c). These results show that our patient-derived iPSCs possess the potential to differentiate into mesenchymal cell lineages, thereby offering the potential for autologous as well as allogeneic MSC transplantation.
Differentiation of autoimmune disease (AID)-specific induced pluripotent stem cells (iPSCs) into mesenchymal lineages. (a) A schematic diagram of the protocol used to obtain multipotent mesenchymal stem cells (MSCs) and terminal mesenchymal cells from patient-specific iPSCs derived from patients with ankylosing spondylitis (AS), Sjögren syndrome (SS) and systemic lupus erythematosus (SLE). (b) Fluorescence-activated cell sorting (FACS) analysis of AID specific-derived MSCs. After 21 days of differentiation, the cells were analyzed by flow cytometry for the expression of CD90 and CD73. The data are presented as mean (s.e.m.) of three individual experiments. (c) Staining of differentiated cells to identify adipocytes (Oil red O), chondrocytes (Alcian blue) and osteocytes (Alizarin red S).
Discussion
The generation of iPSCs from patients with AIDs has been reported in only a few studies, for example, in cases of type 1 diabetes and SLE.15, 22 However, iPSC lines have been generated using genome integrating methods such as retrovirus for type 1 diabetes and lentivirus for SLE, raising concerns regarding the risk of insertional mutagenesis and continued expression of oncogenic proteins after transgene integration.19 These concerns are especially important when considering clinical and translation research. To date, several integration-free methods have been developed, including mRNA,23 Sendai virus,24 recombinant proteins,25 episomal vectors26 and miRNA27 systems; each of these methods has its own advantages and disadvantages.19 Among the available integration-free methods, the reprogramming method using an oriP/EBNA-1-based episomal vector is highly suitable for clinical applications. Because this method is very simple, with no need for repeated transfection and the oriP/EBNA-1-based episomal vector has a wide host cell range,26 it can be used in many human cell types. However, despite these advantages, episomal vector reprogramming approaches have previously resulted in extremely low reprogramming efficiency (~0.0003% for human fibroblasts).28
In this study, we describe the successful reprogramming of cells from patients with AIDs such as AS, SS and SLE using nonintegrating oriP/EBNA-1-based episomal vectors. The addition of 1 mM nicotinamide to the cell cultures improved the reprogramming efficiencies 30- to 220-fold greater than the efficiencies described in our previous study.21 The pluripotency of established AID-specific iPSCs was confirmed through the expression of ESC markers and the capacity of the cells to differentiate into multiple cell lineages in vitro and in vivo. Our results demonstrate the utility of the episomal vector system for generating AID-specific, transgene-free iPSCs that will provide a clinically safe regenerative platform for patient-tailored approaches to AID research.
Hematopoietic stem cell transplantation and mesenchymal stem cell transplantation offer emerging therapies for patients with severe AID. Autologous and allogeneic HSCs and MSCs that have been isolated from patients have been applied in a range of immune-mediated conditions, including AS, SS and SLE.29, 30 However, this approach has limitations because of the limited availability of cell sources and the frequent occurrence of immune rejection. Autologous transplantation from patient-specific iPSC-derived differentiated cells offers a solution to these problems. The unlimited proliferative capacity of iPSCs can provide sufficient quantities of cells for cell therapy. Moreover, the use of patient-specific iPSCs can minimize immune rejection responses. Therefore, iPSC-based therapy may permit the development of new treatments for AID.
To our knowledge, the data reported in this paper provide the first demonstration that AID-specific iPSCs can be obtained from patients with AS, SS and SLE using the nonintegrating episomal method and that established AID-specific iPSCs can be differentiated into representative cell types from all three germ layers both in vitro and in vivo. Most importantly, AID-specific iPSCs possess hematopoietic and mesenchymal differentiation potential; thus, they allow cell replacement therapy by serving as a source of autologous or allogeneic cells and of cells for customized tissue repair in AS, SS and SLE (Figure 6).
Therapeutic potential of patient-specific induced pluripotent stem cells (iPSCs) for the treatment of autoimmune disease. Autologous skin fibroblasts or other cell types from autoimmune disease (AID) patients can be reprogrammed into iPSCs. The defective gene in iPSCs can be corrected by homologous recombination-mediated gene conversion. Hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) derived from patient-specific iPSCs would provide alternative autologous or allogeneic cell sources for cell replacement therapies, such as hematopoietic stem cell transplantation and mesenchymal stem cell transplantation.
A number of obstacles must be overcome before the successful clinical application of AID-specific iPSCs. The clinical application of iPSCs and of the differentiated cells derived from them by culturing with animal-derived products, such as animal cell feeder layers and serum, are hampered by the risk of potential exposure to xeno-pathogens. Thus, culture methods for the generation of iPSCs and the in vitro differentiation of cells required for transplantation that utilize only defined factors that are free of animal-derived components are essential. In the present study, we have obtained in vitro differentiation of AID-specific iPSCs using a defined serum-free and feeder-free method. We successfully differentiated AS-iPSCs, SS-iPSCs and SLE-iPSCs into HSCs using such a system, whereas the method we used to induce mesenchymal lineage differentiation still includes animal-derived products such as fetal bovine serum. Our goal is to develop a xeno-free culture method for the clinical application of AID-specific iPSCs in cell transplantation therapy. In addition, improved differentiation protocols are needed to obtain pure populations of functional cells.
In conclusion, our results demonstrate the successful generation of dermal fibroblast-derived iPSCs from patients with AIDs such as AS, SS and SLE through the use of nonintegrating episomal vectors. AID-specific, transgene-free iPSCs showed the capacity to differentiate into hematopoietic and mesenchymal cell lineages, thereby providing a novel, autologous, patient-specific platform for the therapeutic applications of iPSCs.
References
Lis J, Jarzab A, Witkowska D . [Molecular mimicry in the etiology of autoimmune diseases]. Postepy Hig Med Dosw (Online) 2012; 66: 475–491.
Perl A . Pathogenesis and spectrum of autoimmunity. Methods Mol Med 2004; 102: 1–8.
Taylor CJ, Bolton EM, Bradley JA . Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci 2011; 366: 2312–2322.
Rosa SB, Voltarelli JC, Chies JA, Pranke P . The use of stem cells for the treatment of autoimmune diseases. Braz J Med Biol Res 2007; 40: 1579–1597.
Uccelli A, Prockop DJ . Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr Opin Immunol 2010; 22: 768–774.
Parker MH, Kuhr C, Tapscott SJ, Storb R . Hematopoietic cell transplantation provides an immune-tolerant platform for myoblast transplantation in dystrophic dogs. Mol Ther 2008; 16: 1340–1346.
Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA . Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 2010; 5: 933–946.
Gage F . The promise and the challenge of modelling human disease in a dish. EMBO Mol Med 2010; 2: 77–78.
Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 2009; 5: 111–123.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861–872.
Nishikawa S, Goldstein RA, Nierras CR . The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol 2008; 9: 725–729.
Malik N, Rao MS . A review of the methods for human iPSC derivation. Methods Mol Biol 2013; 997: 23–33.
Ikeda T, Hirata S, Takamatsu K, Haruta M, Tsukamoto H, Ito T et al. Suppression of Th1-mediated autoimmunity by embryonic stem cell-derived dendritic cells. PLoS ONE 2014; 9: e115198.
Li M, Ikehara S . Stem cell treatment for type 1 diabetes. Front Cell Dev Biol 2014; 2: 9.
Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA 2009; 106: 15768–15773.
Son MY, Kwak JE, Seol B, Lee da Y, Jeon H, Cho YS . A novel human model of the neurodegenerative disease GM1 gangliosidosis using induced pluripotent stem cells demonstrates inflammasome activation. J Pathol 2015; 237: 98–110.
Son MY, Seol B, Han YM, Cho YS . Comparative receptor tyrosine kinase profiling identifies a novel role for AXL in human stem cell pluripotency. Hum Mol Genet 2014; 23: 1802–1816.
Son MY, Choi H, Han YM, Cho YS . Unveiling the critical role of REX1 in the regulation of human stem cell pluripotency. Stem Cells 2013; 31: 2374–2387.
Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S et al. A more efficient method to generate integration-free human iPS cells. Nat Methods 2011; 8: 409–412.
Salvagiotto G, Burton S, Daigh CA, Rajesh D, Slukvin II, Seay NJ . A defined, feeder-free, serum-free system to generate in vitro hematopoietic progenitors and differentiated blood cells from hESCs and hiPSCs. PLoS ONE 2011; 6: e17829.
Son MJ, Son MY, Seol B, Kim MJ, Yoo CH, Han MK et al. Nicotinamide overcomes pluripotency deficits and reprogramming barriers. Stem Cells 2013; 31: 1121–1135.
Chen Y, Luo R, Xu Y, Cai X, Li W, Tan K et al. Generation of systemic lupus erythematosus-specific induced pluripotent stem cells from urine. Rheumatol Int 2013; 33: 2127–2134.
Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010; 7: 618–630.
Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 2010; 7: 11–14.
Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009; 4: 381–384.
Yu J, Chau KF, Vodyanik MA, Jiang J, Jiang Y . Efficient feeder-free episomal reprogramming with small molecules. PLoS ONE 2011; 6: e17557.
Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 2011; 8: 376–388.
Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009; 324: 797–801.
Pasquini MC, Voltarelli J, Atkins HL, Hamerschlak N, Zhong X, Ahn KW et al. Transplantation for autoimmune diseases in North and South America: a report of the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 2012; 18: 1471–1478.
Figueroa FE, Carrion F, Villanueva S, Khoury M . Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res 2012; 45: 269–277.
Acknowledgements
This work was supported by the NRF Grant (2012M3A9C7050224) and by the NST Grant (CRC-15-02-KRIBB) from the Korean government (MSIP). The funders had no role in the study design, data collection or analysis, decision to publish or preparation of the manuscript.
Author contributions
M-YS and YSC designed the studies. M-YS performed the molecular and differentiation experiments, data analysis and wrote the manuscript. M-OL carried out HSC and MSC differentiation and flow cytometry characterization and wrote the manuscript. HJJ and BS cultured iPSCs, performed experiments for iPSC characterization and helped to write the manuscript. JHK cultured primary cells and helped to write the manuscript. J-SC designed the experiments, analyzed the data and wrote the manuscript. YSC planned the project and critically revised the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Son, MY., Lee, MO., Jeon, H. et al. Generation and characterization of integration-free induced pluripotent stem cells from patients with autoimmune disease. Exp Mol Med 48, e232 (2016). https://doi.org/10.1038/emm.2016.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2016.27
This article is cited by
-
Gene delivery in adherent and suspension cells using the combined physical methods
Cytotechnology (2022)
-
Stem cells: a potential treatment option for kidney diseases
Stem Cell Research & Therapy (2020)
-
Induction of a local muscular dystrophy using electroporation in vivo: an easy tool for screening therapeutics
Scientific Reports (2020)
-
Modelling cardiac fibrosis using three-dimensional cardiac microtissues derived from human embryonic stem cells
Journal of Biological Engineering (2019)
-
The Post-GWAS Era: How to Validate the Contribution of Gene Variants in Lupus
Current Rheumatology Reports (2019)