Abstract
Periodontitis is a common chronic inflammatory disease. Recent studies have shown that chronic stress (CS) might modulate periodontal disease, but there are few models of CS-induced periodontitis, and the underlying mechanisms are unclear. The present study established a rat model of periodontitis associated with CS induced by nylon thread ligatures. The severity of periodontitis was evaluated in this model by radiographic and pathological examination. The inflammatory reaction indicated by the elevated serum levels of interleukin (IL)-1β, IL-6 and IL-8 was assessed by enzyme-linked immunosorbent assay. Toll-like receptor-4 (TLR4) and glucocorticoid receptor-α (GR-α) expressions were detected by reverse transcriptase-PCR and western blotting. Open-field tests and serum corticosterone were used to evaluate CS. The results showed that CS induced behavioral changes and increased corticosterone levels of the animals with periodontitis. CS stimulation markedly increased alveolar bone loss, periodontal pocket depth and the number of plaques. It also enhanced the inflammatory reaction. These results suggest that CS accelerated the ligature-induced pathological changes associated with periodontitis. Further analysis of the mechanisms involved showed that GR-α expression was significantly downregulated in periodontal tissues of the animals undergoing CS. Blocking GR-α signaling in lipopolysaccharide and corticosteroid-treated human periodontal ligament fibroblast cells in vitro significantly upregulated the expression of p-Akt (protein kinase B) and TLR4, promoted nuclear factor-κB activity and increased levels of IL-1β, IL-6 and IL-8. This research suggests that CS might accelerate the pathological progression of periodontitis by a GR-α signaling-mediated inflammatory response and that this may be a potential therapeutic target for the treatment of periodontal disease, particularly in patients with CS.
Similar content being viewed by others
Introduction
Periodontitis is a multifactorial disease characterized by the destruction of the supporting structures of the teeth (the periodontal ligament and alveolar bone), and it affects millions of people each year.1 Studies have shown that the identification of risk factors is increasingly important for the treatment and prevention of periodontitis.2 Studies from multiple countries have demonstrated that 7 to 15% of the dentate adult population suffer from destructive periodontal disease.3 In addition to the effects of bacteria, which are found on the surface of the tooth and on the tooth root,4 there is also strong evidence showing that the destructive processes occurring as part of the host inflammatory response are responsible for the majority of the hard and soft tissue breakdown that leads to clinical signs of periodontitis.5
There are many environmental risk factors that are associated with the inflammatory response. These risk factors, including smoking, diabetes mellitus and poor health habits, are important components driving the chronic inflammatory response in periodontitis.6 Factors such as stress, depression and anxiety, all of which have long been linked to periodontal disease, are also frequently identified as additional factors that may affect the progression of periodontal disease.7, 8
Chronic stress (CS) is an internal response by the hypothalamic–pituitary–adrenal (HPA) axis to a prolonged period of emotional or physiological stress.9 It involves a release of corticosteroids by the endocrine system. Although the immediate effects of stress hormones are beneficial in situations of acute stress, exposure to long-term stress results in high levels of these hormones and may lead to high blood pressure, damage to muscle tissue, inhibition of growth and suppression of the immune system.10 These effects may ultimately contribute to the exacerbation of periodontitis. The idea that chronic stress fosters disease by activating the HPA axis is an important component of understanding the progression of a disease. In the past decade, evidence from epidemiologic studies has confirmed that there is a correlation between periodontitis and stress, depression and negative life events.11 Studies of the effects of CS commonly restrain rats for 12 h on 1 day as a way to model CS. However, after 2 h of continuous exposure to immobilization, stress will significantly impair the HPA axis response.12 Therefore, it is important to establish a more effective animal model of CS-induced periodontitis. Although there is a clear relationship between stress and periodontal disease, information on exactly how to define CS and whether CS may accelerate the pathological progression of periodontitis is unclear. The present study evaluated the individual effects of ligation, chronic stress and their combined effects on the progression of periodontitis in rats to assess a new animal model of CS-induced periodontitis. The effects on body weight, serum levels of corticosterone, behavioral changes and the pathological progression of periodontitis were analyzed. In addition, the mechanism responsible for these effects was analyzed.
Materials and methods
Animals
A total of 120 male Wistar rats (Charles River Laboratories, Wilmington, MA, USA) with an average initial weight of 180 g were selected. The animals were housed at a stable temperature (20–24 °C) with a relative humidity of 50–70% and a 12-h light/dark cycle. Animals were acclimatized to the housing conditions over 4 weeks. Food and water were available ad libitum except during times when restraint or food/water deprivation stresses were applied. Because crowding or isolation may be stressful to experimental animals,13 animals were housed five per cage throughout the experiment. The animals were weight stratified and then randomly assigned to one of the four experimental groups: control group (G1), chronic stress group (G2), ligature-induced periodontitis group (G3) and ligature-induced periodontitis with chronic stress group (G4). Each group contained 30 rats.
Experimental periodontitis
All procedures were in agreement with the standards for the care of laboratory animals and were approved by the Medical Ethics Committee of the National Institutes of Health guidelines. Experimental periodontitis was induced in G3 and G4 animals as described by Kayal.14 Ligation was performed under general anesthesia (0.5 ml kg−1 intramuscular administration of a combination of ketamine and xylazine; Ketanest, Parke-Davis, Berlin, Germany). After anesthesia, the right maxillary first molars of each animal were ligated with 0.20 mm nylon thread (Ethicon, Johnson & Johnson, São Paulo, Brazil) placed around the neck of tooth in the gingival area to induce experimental periodontal disease. G1 and G2 animals were not ligated. The ligatures were kept in place for the entire experimental period and served as a way to accumulate oral microorganisms. After the operation, rats from G3 and G4 received wet food and a syrup drink (100 ml water with 10 g sugar) for 3 days.
Chronic stress induction
Chronic stress was induced daily in G2 and G4 from 0830 to 1030 or from 1430 to 1630 h starting on the fourth day after the induction of periodontitis using a modification of the methods of Willner et al.15 The experimental period lasted 4 weeks. During each week of the stress regime, rats underwent two periods of each of the following treatments: food or water deprivation; 45° cage tilt; soiled cage (250 ml water added to the sawdust bedding); feet shocking (5 min); swimming in 4 °C or 45 °C water (5/min); tail clamp (1 min); being bound; and no stress. All treatments were applied randomly. All groups were weighed, and open-field activity was assessed after 1, 10, 20 and 30 days of the experiment.
Open-field test
Behavioral changes in rats were determined by the open-field test. The open-field test is a common measure of exploratory behavior and general activity in rats, where both the quality and quantity of the activity can be measured.16 After 1, 10, 20 and 30 days of CS induction, behaviors were assessed from 800 to 1000 h using an open-field test. The rats were placed in an open field (Xinruan, Shanghai, China), which was 100 cm × 100 cm × 50 cm, with black walls and a bottom composed of 25 equal sections. The number of times the rats crossed through the center of the grid was quantified during a 3-min period, as was the frequency of rearing.
Enzyme-linked immunosorbent assay (ELISA)
At the end of experiment, animals were anesthetized, and blood was collected in centrifuge tubes. Blood samples were centrifuged at 3000 revolutions per min for 10 min at 4 °C. Serum was extracted, stored in plastic tubes and frozen at −20 °C to assess the levels of corticosterone, interleukin (IL)-1β, IL-6 and IL-8. The concentrations of alanine transaminase, glucose, corticosterone, IL-1β, IL-6 and IL-8 in both serum and culture medium were assayed using ELISA kits (Abcam, Cambridge, MA, USA) according to the manufacturer’s recommendations. Duplicate aliquots were used for the analyses.
Periodontitis evaluation
The severity of periodontitis was evaluated using the gingival index, periodontal pocket depth, plaque index and tooth mobility. Probing depth was established by inspecting the soft gum tissue around the teeth with a probe. Bone loss was detected by both conventional radiography and histometric evaluation.
Sample collection
All animals were killed by an overdose of ether at the end of the experiment. The marginal gingival tissues containing alveolar bone and the soft tissues surrounding the maxillary first molars were collected. All tissue samples were fixed in 4% buffered formaldehyde (pH 7.4) for 48 h. Radiographs were taken using a dental X-ray unit (Specto70x, DabiAtlante, Ribeirao Preto, Brazil) to determine alveolar bone loss in ligated and nonligated teeth. Inflammatory cells, osteoclasts, fibers and fibroblasts were counted in the connective tissue of all experimental groups. In addition, the tissues were sectioned for histometric evaluation.
Histological procedures
The tissue blocks from the maxilla were perfused with phosphate-buffered saline and then with 4% paraformaldehyde for 48 h under physiological pressure. They were decalcified in trichloroacetic acid until complete decalcification was radiographically confirmed. After dehydration in a graded alcohol series, the samples were embedded in paraffin and cut into 6 μm serial sections. Corresponding sections were stained with hematoxylin (Sigma, St Louis, MO, USA) for 10 min at room temperature. Sections were then stained with eosin (0.5%, Merek, Whitehouse Station, NJ, USA) for 30 s, washed with running water and covered for observation. Sections were observed using Image-Pro Plus 5.0 software (Media Cybernetics, Bethesda, MD, USA) (200 ×) for lesion area analysis.
Reverse transcription-PCR
Total RNA was extracted from the periodontal tissue and the cultured cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. After assessing RNA purity, ∼5 μg total RNA from each sample was reverse transcribed into first-strand complementary DNA (cDNA) for reverse transcription-PCR analysis. The specific primers used were as follows: Toll-like receptor-4 (TLR4; rat), 5′-AATGAGGACTGGCTGAC-3′ (forward primer) and 5′-TGAGGTCGTTGAGCTTAG-3′ (reverse primer); TLR4 (human), 5′-TGATGTCTGCCTCGCGCCTG-3′ (forward primer) and 5′-TAGGACCACCTCCACGCAGGG-3′ (reverse primer); β-actin (rat), 5′-ACCACAGCTGAGAGCGAAATCG-3′ (forward primer) and 5′-AGAGGTCTTTACGGATCTCAACG-3′ (reverse primer); and β-actin (human), 5′-ACTCGTCATACTCCTGCT-3′ (forward primer) and 5′-GAAACTACCTTCAACTCC-3′ (reverse primer). The amplification program was 94 °C for 3 min, followed by 33 cycles of 94 °C for 1 min, 58 °C for 1 min and 72 °C for 1.5 min, followed by a final step at 72 °C for 15 min. Aliquots of the PCR products were electrophoresed on 1.5% agarose gels, and PCR fragments were visualized by ethidium bromide staining. Real-time PCR experiments for each gene were performed in triplicate. β-Actin was used as the internal control for mRNA.
Western blotting analysis
Periodontal tissue and cells were homogenized and lysed in RIPA lysis buffer (100 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 1 mM EDTA, 10 mM β-glycerophosphate, 2 mM sodium vanadate and protease inhibitor). Protein concentration was assayed using the micro-BCA protein assay (Pierce, Rockford, IL,USA). Proteins (40 μg per lane) were separated by 12% SDS–polyacrylamide gel electrophoresis and electroblotted onto a nitrocellulose membrane (Amersham Pharmacia, Freiburg, Germany). Nonspecific binding was blocked by incubating membranes with 5% non-fat milk in Tris-buffered saline with Tween-20 buffer at room temperature for 1 h. The nitrocellulose membranes were incubated with anti-p-Akt (1:500), anti-TLR4 (rat/human) (1:500), anti-glucocorticoid receptor-α (GR-α) (rat/human) (1:1000) or anti-β-actin (rat/human) (1:500) antibodies for 1 h at room temperature. Following three washes with Tris-buffered saline containing Tween-20, horseradish peroxidase-conjugated secondary antibodies were added, and enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ, USA) was used for detection.
HPDLF isolation, culture and characterization
Human periodontal ligament fibroblasts (HPDLFs) were isolated from the middle third region of the roots of periodontally healthy teeth of adolescents during standard orthodontic procedures at the PLA Navy General Hospital of Dentistry after obtaining the donors’ informed consent. The cells were cultured and characterized as described previously.17 HPLF2630 cells were purchased from SHBIO (Sixin, Shanghai, China) and cultured following the manufacturer’s protocol.
SiRNA transfection
GA-α small interfering RNA (siRNA; Sigma-Aldrich) transfection was performed according to the manufacturer’s instruction. Briefly, 1 μg of vector was incubated with 5.0 μl of serum-free medium for 5 min (Solution A), and 2 μl of Lipofectamine 2000 (Invitrogen) was incubated with serum-free medium for 5 min (Solution B). Solution A was mixed with solution B and incubated for 20 min. After incubation, HPDLF cells were added to the mixture. Cells were then harvested at 24 h after transfection for further analysis. The efficiency of GA-α siRNA was confirmed by western blotting analysis.
GA-α plasmid construction and cell transfection
Total RNA was isolated from HPDLFs using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. The cDNA was synthesized by reverse transcription of total RNA using the PrimeScript RT reagent kit (Takara, Dalian, China) with oligo-dT primers according to the manufacturer’s protocol. The open reading frame of GA-α cDNA was then cloned and inserted into the pcDNA3.1 vector (Invitrogen) to create pcDNA3.1-GA-α expression vectors. Cells transfected with the pcDNA3.1 vector alone were used as negative controls. Cell transfection was performed using the FuGENE HD transfection reagent (Roche, Indianapolis, IN, USA) method as suggested by the manufacturer.
NF-κB activity assays
Treated cells were co-transfected with pNifty-Luc NF-κB-Luciferase reporter cDNA and β-galactosidase cDNA using Lipofectamine 2000, following the manufacturer’s guidelines, and cultured for 24 h. Transfected cells were then incubated in the absence or presence of lipopolysaccharide (LPS; 20 ng ml−1) for 3 h. Luciferin (0.15 mg ml−1) was then added to each well, and luminescence was measured using a FLUOstar Omega (BMG Labtech, Offenburg, Germany) plate reader. Cells were washed with phosphate-buffered saline and incubated with a β-galactosidase assay reagent for 30 min. Absorbance at 405 nm was measured using a colorimetric plate reader. Nuclear factor-κB (NF-κB) activity was defined as the luminescence normalized to the optical density of the colorimetric detection of β-galactosidase.
Statistical analysis
The results are presented as mean±s.d. Analyses among three or four groups were carried out by the Tukey–Kramer test in cases where a significant effect was shown by the one-way analysis of variance F-test. Statistical analyses for direct two-group comparisons were performed with the unpaired t-test, and statistical analyses to compare changes within one group before and after the experimental process were performed with the paired t-test. Analyses were conducted using SPSS13.0 software (Chicago, DE, USA). A difference was considered statistically significant at P<0.05.
Results
An animal model of periodontitis with chronic stress
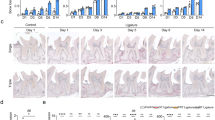
The mean body weight of all rats was ∼180 g at the beginning of the study. There were no statistically significant differences among the groups (P>0.05). Compared with G1, the animals with periodontitis induced by ligature (G3) showed a slower increase in body weight, but the difference was not statistically significant. Furthermore, animals experiencing CS alone (G2) and those undergoing the periodontitis and CS treatment (G4) exhibited slower increases in body weight than in G1 animals. At the end of the treatment, there was a significant difference between G1 and G2 and between G1 and G4 in mean body weight (both P<0.05), but there was no statistically significant difference between G1 and G3. The final mean body weights were 231.4±5.3 g for G1, 215.5±9.2 g for G2, 226.1±8.6 g for G3 and 209.7±10.8 g for G4 (Figure 1a). There were no statistically significant differences among the four groups in serum levels of glucose or alanine transaminase throughout the experimental period (Figures 1b and c).
Chronic stress-induced changes in body weight and behavior of rats. After pretreatment with a ligature and the induction of chronic stress, the body weight of rats was assessed (a). Serum levels of glucose (b) and alanine transaminase (ALT) (c) were detected by enzyme-linked immunosorbent assay (ELISA); the number of grid crossings (d) and rearing frequency (e) were analyzed by an open-field test after 1, 10, 20 and 30 days of treatment. The serum levels of the stress marker corticosterone were determined by an ELISA kit at the end of the experimental period (f). G1, control group (n=30); G2, chronic stress group (n=30); G3, ligature-induced periodontitis group (n=30); and G4, ligature-induced periodontitis with chronic stress group (n=30); *P<0.05 compared with G1, #P<0.05 compared with G3.
After 10 days of CS induction, rats in G2 and G4 began to exhibit abnormal behavioral changes, such as biting each other, being restless and easily frightened and frequently being in a state of high alert. As shown in the open-field test, CS treatment reduced the number of grids crossed by rats (G2 vs G1, and G4 vs G1, both P<0.05) (Figure 1d). Similarly, the frequency of rearing in G2 and G4 was also significant less compared with that of G1 (both P<0.05; Figure 1e).
The HPA axis is the central neuroendocrine pathway for the physiological stress responses in mammals. The glucocorticoid hormone corticosterone, the major stress hormone in rodents, is secreted by the HPA axis when individuals respond to a stressor.18 In our study, after CS treatment, serum levels of corticosterone were significantly increased in G2 and G4 compared with that of G1 (both P<0.05; Figure 1f).
CS enhanced the severity and inflammatory reaction of periodontitis
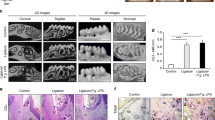
To explore the effects of CS on the progression of periodontitis, the severity of periodontitis and the inflammatory reaction of periodontal tissue were evaluated. An X-ray test of alveolar bone loss showed that greater bone loss was observed in G3 and G4 at 30 days compared with that in the control group (G1). Most importantly, the alveolar bone loss in G4 was significantly increased compared with that in G3 (Figure 2a). The severity of periodontitis was determined based on the clinical indicators gingival index, periodontal pocket depth, plaque index and tooth mobility. Analysis showed that the gingival index of both G3 and G4 were significantly increased compared with that of G1, with the greatest increase observed in G4 (Figure 2b). Analysis of periodontal pocket depth, number of plaques and tooth mobility also showed that ligature placement resulted in more serious clinical manifestations compared with those observed in the nonligated controls. In addition, CS significantly enhanced these clinical manifestations in rats with periodontitis compared with those without periodontitis (Figures 2c–e).
Chronic stress (CS) enhanced the severity and inflammatory reaction of experimental periodontitis. At the end of the experimental period, alveolar bone loss (ABL) was assessed by an X-ray test and quantified (a), and gingival index (GI) (b), periodontal pocket depth (PPD) (c), plaque index (PI) (d) and tooth mobility (e) were analyzed by a probe examination. G1, control group (n=30); G2, chronic stress group (n=30); G3, ligature-induced periodontitis group (n=30); and G4, ligature-induced periodontitis with chronic stress group (n=30); *P<0.05 compared with G1, #P<0.05 compared with G3.
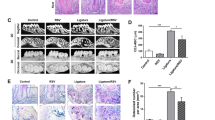
Hematoxylin and eosin staining was used to illustrate the histopathological alteration of periodontal tissues (Figure 3a). The inflammatory reaction was noticeable with ligature treatment (G3). In this group, the organizational structures were damaged, and there was an increased number of osteoclasts and decreased numbers of fibers and fibroblasts (P<0.05) compared with those in the nonligated controls (G1). The inflammatory infiltrates were primarily composed of mononuclear cells indicating a chronic inflammatory reaction. Furthermore, cotreatment with CS (G4) enhanced the inflammatory reaction, increased damage to the periodontal ligament and induced a significant increase in osteoclasts and decreases in fibers and fibroblasts (P<0.05) compared with those in G3. Furthermore, no obvious pathological change in the connective tissues was observed in the CS group (G2) (Figures 3b–e).
Further histologic changes in local tissue were monitored by hematoxylin and eosin (HE) staining. An inflammatory reaction was noticeable, and damage to organizational structures were observed in G3 and G4 (a). Different elements of the connective tissue, including the average number of inflammatory cells (b), osteoclasts (c), fibroblasts (d) and the relative area of collagen fibers (e), were quantified under high magnification. G1, control group (n=15); G2, chronic stress group (n=15); G3, ligature-induced periodontitis group (n=15); and G4, ligature-induced periodontitis with chronic stress group (n=15); *P<0.05 compared with G1, #P<0.05 compared with G3. Scale bar, 100 μm.
CS inhibits GR-α expression in periodontal tissue
The ELISA showed that CS significantly enhanced the increases in IL-1β (Figure 4a), IL-6 (Figure 4b) and IL-8 (Figure 4c) expression brought about by periodontitis. CS also upregulated the expression of TLR4 as shown by an increase in both mRNA and protein levels (Figures 4d and e). These results confirmed that CS can effectively enhance the inflammatory reaction that occurs with periodontitis.
Chronic stress (CS) increased the inflammatory reaction and inhibited glucocorticoid receptor-α (GR-α) expression in periodontal tissue. At the end of the experimental period, periodontal tissues were harvested and the levels of inflammatory-related cytokines interleukin (IL)-1β (a), IL-6 (b) and IL-8 (c) were analyzed by enzyme-linked immunosorbent assay (ELISA). The expression of Toll-like receptor-4 (TLR4) mRNA (d) and protein (e) were analyzed by reverse transcriptase-PCR (RT-PCR) and western blotting. The protein level of GR-α was assessed by western blotting (f). G1, control group (n=15); G2, chronic stress group (n=15); G3, ligature-induced periodontitis group (n=15); and G4, ligature-induced periodontitis with chronic stress group (n=15); *P<0.05 compared with G1, #P<0.05 compared with G3.
GR-α, a transcriptional control factor, is the predominant glucocorticoid receptor isoform. New evidence indicates that the deregulation of GR-α plays an important role in inflammatory disorders19 and that the function of GR-α can be affected by many environmental factors, such as CS.20 To determine whether CS influenced GR-α expression in periodontal tissue or whether it was correlated with the enhanced inflammatory reaction observed in G4, the expression of GR-α in periodontal tissues was assessed. The results showed that the expression of GR-α was significantly decreased in both G2 and G4 compared with that in G1, whereas there was no significant difference in expression between G3 and G1 (Figure 4f). These results suggest that GR-α may be involved in the mechanisms underlying CS-induced increases in the progression of periodontitis.
Effects of GR-α on the LPS-induced inflammatory reaction
HPDLFs are important periodontal ligament cells. They are involved in the synthesis and absorption of collagen and can repair the collagen in the periodontal ligament to promote recovery from periodontitis.21 Therefore, we explored the effect of GR-α on periodontal ligament cells under inflammatory conditions. We pretreated the HPDLF and HPLF2630 cells with 20 ng ml−1 LPS and 100 ng ml−1 corticosteroids for 48 h to simulate the inflammatory environment present during the CS induction of periodontitis.
To determine the effect of GR-α on the LPS-induced inflammatory reaction, the cells were stimulated with LPS and corticosteroids followed by GR-α siRNA or pcDNA3.1-GA-α for 48 h. Western blotting analysis showed that expression of GR-α was significantly decreased after GR-α siRNA pretreatment, whereas pcDNA3.1-GA-α pretreatment significantly increased GA-α expression compared with that of the control (Figure 5a). Interestingly, GR-α siRNA pretreatment significantly promoted the phosphorylation levels of Akt (Figure 5b). Previous reports have shown that Akt phosphorylation contributes to increased TLR4 transcription.22 Furthermore, reverse transcriptase-PCR and western blotting analysis showed a marked increase in TLR4 expression in both HPDLFs and HPLF2630 cells after GR-α siRNA pretreatment and a decrease in TLR4 expression after GR-α overexpression compared with that in the LPS group (Figures 5c and d). As expected, GR-α plays an important role in the LPS-induced inflammatory reaction. Recent evidence indicates that the interaction between GR-α and NF-κB is a key pathogenic reaction in inflammatory disorders.19 An NF-κB activity assay indicated that culturing cells with LPS significantly increased NF-κB activity and that GR-α siRNA pretreatment enhanced this effect, whereas pcDNA3.1-GA-α pretreatment inhibited this effect (Figure 5e). Samples of the culture medium were also collected, and the concentrations of IL-1β, IL-6 and IL-8 were examined. The results showed that LPS significantly increased the levels of IL-1β, IL-6 and IL-8 and that GR-α siRNA pretreatment further increased the levels of IL-1β, IL-6 and IL-8 (Figures 5f–h). However, GA-α overexpression effectively eliminated the LPS-induced increases in the levels of IL-1β, IL-6 and IL-8. These result suggest that GR-α may inhibit the LPS-induced inflammatory reaction by affecting TLR4 expression and suppressing the activity of NF-κB.
Effects of glucocorticoid receptor-α (GR-α) on the lipopolysaccharide (LPS)-induced inflammatory reaction. To clarify the underlying mechanisms of this effect, GR-α small interfering RNA (siRNA) and pcDNA3.1-GR-α were introduced to silence GR-α signaling. Following pretreatment with 20 ng ml−1 LPS and 100 ng ml−1 corticosteroids (GCs) and GR-α siRNA/pcDNA3.1-GR-α for 48 h, the protein levels of GR-α (a) and p-Akt (b) were assessed by western blotting. Toll-like receptor-4 (TLR4) mRNA (c) and protein (d) expressions were also detected, and nuclear factor (NF)-κB activity was assessed by Luciferase assay (e). The LPS-induced release of interleukin (IL)-1β (f), IL-6 (g) and IL-8 (h) was analyzed by enzyme-linked immunosorbent assay (ELISA). *P<0.05 compared with control, #P<0.05 compared with (LPS+mock) or (LPS+blank). Mock, scramble siRNA used as the control for GR-α siRNA; blank, pcDNA3.1 without GR-α gene sequence used as the control for pcDNA3.1-GR-α.
Discussion
Periodontitis is a multifactorial disease that affects the periodontium, the tissues surrounding and supporting the teeth.23 A diagnosis of periodontitis is established during clinical examination by inspecting the soft gum tissue around the teeth with a probe and evaluating the patient’s X-ray films to determine the amount of bone loss around the teeth.24 In this study, placing a nylon thread ligature around the neck of the maxillary first molar of rats for 30 days resulted in bleeding and erosion in the soft gum tissues and the formation of a periodontal pocket. Bone loss around the teeth, as shown by X-ray examination and histopathological evaluation, the periodontal pocket depth and the number of plaques present were all greater in the groups with periodontitis. These results demonstrated a successful establishment of a model of periodontitis that had a clinical course similar to that in humans.
Stress may be defined as a psychophysiological response state of an organism facing the perception of a challenge or a threat to the body. Stress plays an important role in the etiology and maintenance of many inflammatory diseases, including periodontal disease.25 However, because of the difficulties in quantifying the amount and duration of stress or the possible adaptive responses to stress, the evaluation of the criteria needed to study chronic stress in an animal model of periodontal disease remains incomplete. In the present study, we provided multiple, random stressful stimuli for 2–4 h a day to reduce the ability of the rats to adapt to chronic stress. We used body changes in weight, open-field test behaviors and serum corticosterone levels to evaluate the effects of CS.
Although our results demonstrated significant differences in body weight, the number of grid crossings and rearing frequency between the CS groups and the control group, there were no differences between the groups of animals without CS. Analysis of serum corticosterone levels also showed a statistically significant difference between the CS and control group. These results indicate that a suitable model was developed for the study of the effects of CS on periodontitis. Acute stress has been reported to have a negative effect on blood glucose concentrations in patients with type 1 and type 2 diabetes.26 Therefore, we also tested whether our model of chronic stress affected the general health of the animals. There was no difference in ALP or glucose levels among the four groups throughout the experimental period.
Using the animal models described above, we analyzed the effects of CS on the progression of periodontitis. Previous studies have suggested that chronic stress may have a net negative effect on the immunological response of the body, leading to an imbalance between hosts and parasites and resulting in further periodontal breakdown.27, 28 Several studies have investigated the possible relationship between psychological stress and periodontitis in rats and have suggested that stress may play a role in the development of periodontal disease.29, 30 In the present study, we showed that CS applied over a period of 4 weeks accelerates the progression of ligature-induced periodontitis, as shown by clinical indications, histological changes and radiographic examination. CS in conjunction with periodontitis also increased serum levels of IL-1β, IL-6 and IL-8 and increased the expression of TLR4 in periodontal tissue. However, no significant differences were observed in the structure of the periodontal tissues between the group undergoing only the CS treatment and the control group. These results indicate that CS alone does not induce any significant changes in periodontal tissues, but that it can significantly accelerate the pathological progression of existing periodontal inflammation and result in the accelerated degradation of periodontal tissues.
GR-α is the predominant isoform of glucocorticoid receptors to which glucocorticoids bind to exert anti-inflammatory and immunosuppressive actions. GR-α expression has been measured in a number of inflammatory disorders and may play a major role in the impaired inflammation response associated with these diseases.31, 32 More importantly, recent studies have shown that impaired HPA axis activity may be associated with glucocorticoid resistance.20 In this study, the GR-α expression was significantly downregulated in the animals that received CS treatment. Further analysis of the mechanisms responsible for this response show that GR-α siRNA administration significantly enhanced Akt phosphorylation and thus promoted TLR4 transcription. GR-α silencing also promoted LPS-induced NF-κB activation and increased the secretion of IL-1β, IL-6 and IL-8. GR-α overexpression markedly decreased the changes brought about by LPS. Interestingly, we found that CS accelerated the inflammatory reaction only under conditions where periodontitis was present and did not induce inflammatory reactions under other conditions. This may because of the fact that balance of the immune system is more easily disrupted when disease is present, or there may be other pathways that take part in the GR-α-mediated phosphorylation of Akt. These possibilities will be further investigated in our future studies.
In conclusion, the present study demonstrated a more effective animal model for studying periodontal disease in association with chronic stress and confirmed that CS treatment accelerates the inflammatory response and tooth damage cause by periodontitis. Analysis of the mechanism responsible for this response showed that CS may accelerate the progression of periodontitis through effects on GR-α signaling pathways. Thus, our study provides novel insight into the mechanism by which CS accelerates the pathological progression of periodontitis and may facilitate the development of a therapeutic strategy, such as GR-α-regulating drugs for patients with both CS and periodontal disease.
References
Darveau RP . Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 2010; 8: 481–490.
Menon SM, Perayil J . Relationship between life event stress and periodontitis and its response to treatment. Int J Contemp Dent 2012; 3: 10–13.
Johnson N, Griffiths G, Wilton J, Maiden M, Curtis M, Gillett I et al. Detection of high-risk groups and individuals for periodontal diseases. J Clin Periodontol 1988; 15: 276–282.
Zarco MF, Vess TJ, Ginsburg GS . The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis 2012; 18: 109–120.
Deo V, Bhongade ML . Pathogenesis of periodontitis: role of cytokines in host response. Dent Today 2010; 29: 60–62, 64–66; quiz 68-69.
Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K et al. Periodontitis and diabetes: a two-way relationship. Diabetologia 2012; 55: 21–31.
Rosania AE, Low KG, McCormick CM, Rosania DA . Stress, depression, cortisol, and periodontal disease. J Periodontol 2009; 80: 260–266.
Sabbah W, Tsakos G, Chandola T, Newton T, Kawachi I, Sheiham A et al. The relationship between social network, social support and periodontal disease among older Americans. J Clin Periodontol 2011; 38: 547–552.
Gaspersic R, Stiblar-Martincic D, Skaleric U . Influence of restraint stress on ligature-induced periodontitis in rats. Eur J Oral Sci 2002; 110: 125–129.
Dhabhar FS . Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009; 16: 300–317.
Genco RJ, Borgnakke WS . Risk factors for periodontal disease. Periodontol 2000 2013; 62: 59–94.
Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM et al. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 2011; 35: 1291–1301.
Herzog CJ, Czeh B, Corbach S, Wuttke W, Schulte-Herbruggen O, Hellweg R et al. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience 2009; 159: 982–992.
Kayal RA . The role of osteoimmunology in periodontal disease. BioMed Res Int 2013; 2013: 639368.
Willner P, Muscat R, Papp M . Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 1992; 16: 525–534.
Gould TD, Dao DT, Kovacsics CE . The open field test. Mood and Anxiety Related Phenotypes in Mice 2009; 42: 1–20.
Lu H, Xu M, Wang F, Liu S, Gu J, Lin S . Chronic stress enhances progression of periodontitis via alpha1-adrenergic signaling: a potential target for periodontal disease therapy. Exp Mol Med 2014; 46: e118.
Qulu L, Daniels WM, Mabandla MV . Exposure to prenatal stress has deleterious effects on hippocampal function in a febrile seizure rat model. Brain Res 2015; 1624: 506–514.
Lin XC, Sun HY, Zhen YX, Zhang H, Shi H, Wang XX . Low expression of glucocorticoid receptor a in oral lichen planus correlates with activation of nuclear factor kappaB: a preliminary study. J Oral Pathol Med 2014; 43: 600–605.
Silverman MN, Sternberg EM . Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann NY Acad Sci 2012; 1261: 55–63.
El-Awady AR, Messer RL, Gamal AY, Sharawy MM, Wenger KH, Lapp CA . Periodontal ligament fibroblasts sustain destructive immune modulators of chronic periodontitis. J Periodontol 2010; 81: 1324–1335.
Kim SY, Jeong E, Joung SM, Lee JY . PI3K/Akt contributes to increased expression of Toll-like receptor 4 in macrophages exposed to hypoxic stress. Biochem Bioph Res Co 2012; 419: 466–471.
Savage A, Eaton KA, Moles DR, Needleman I . A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol 2009; 36: 458–467.
Lin KC, Wadhwani CP, Sharma A, Finzen F . A radiograph positioning technique to evaluate prosthetic misfit and bone loss around implants. J Prosthet Dent 2014; 111: 163–165.
Akcali A, Huck O, Tenenbaum H, Davideau JL, Buduneli N . Periodontal diseases and stress: a brief review. J Oral Rehabil 2013; 40: 60–68.
Marcovecchio ML, Chiarelli F . The effects of acute and chronic stress on diabetes control. Sci Signal 2012; 5: 1–10.
Stabholz A, Soskolne WA, Shapira L . Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontol 2000 2010; 53: 138–153.
Reddy S, Kaul S, Prasad M, Agnihotri J, Amudha D, Vinayak R . Interlink between stress and periodontal disease. Health Renaissance 2012; 10: 126–131.
Gomes EP, Aguiar JC, Fonseca-Silva T, Dias LC, Moura-Boas KP, Roy A et al. Diazepam reverses the alveolar bone loss and hippocampal interleukin-1beta and interleukin-6 enhanced by conditioned fear stress in ligature-induced periodontal disease in rats. J Periodontal Res 2013; 48: 151–158.
Huang S, Lu F, Zhang Z, Yang X, Chen Y . The role of psychologic stress-induced hypoxia-inducible factor-1alpha in rat experimental periodontitis. J Periodontol 2011; 82: 934–941.
Barnes PJ . Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immun 2013; 131: 636–645.
Sun XJ, Li ZH, Zhang Y, Zhou G, Zhang JQ, Deng JM et al. Combination of erythromycin and dexamethasone improves corticosteroid sensitivity induced by CSE through inhibiting PI3K-delta/Akt pathway and increasing GR expression. Am J Physiol Lung C 2015; 309: 139–146.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81070837) and National High Technology Research and Development Program 863 (No. 2013AA032201).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Lu, H., Xu, M., Wang, F. et al. Chronic stress accelerates ligature-induced periodontitis by suppressing glucocorticoid receptor-α signaling. Exp Mol Med 48, e223 (2016). https://doi.org/10.1038/emm.2015.127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2015.127
This article is cited by
-
Psychological stress: neuroimmune roles in periodontal disease
Odontology (2023)