Abstract
Recent evidence has suggested that human skin fibroblasts may represent a novel source of therapeutic stem cells. In this study, we report a 3-stage method to induce the differentiation of skin fibroblasts into insulin-producing cells (IPCs). In stage 1, we establish the isolation, expansion and characterization of mesenchymal stem cells from human labia minora dermis-derived fibroblasts (hLMDFs) (stage 1: MSC expansion). hLMDFs express the typical mesenchymal stem cell marker proteins and can differentiate into adipocytes, osteoblasts, chondrocytes or muscle cells. In stage 2, DMEM/F12 serum-free medium with ITS mix (insulin, transferrin, and selenite) is used to induce differentiation of hLMDFs into endoderm-like cells, as determined by the expression of the endoderm markers Sox17, Foxa2, and PDX1 (stage 2: mesenchymal-endoderm transition). In stage 3, cells in the mesenchymal-endoderm transition stage are treated with nicotinamide in order to further differentiate into self-assembled, 3-dimensional islet cell-like clusters that express multiple genes related to pancreatic β-cell development and function (stage 3: IPC). We also found that the transplantation of IPCs can normalize blood glucose levels and rescue glucose homeostasis in streptozotocin-induced diabetic mice. These results indicate that hLMDFs have the capacity to differentiate into functionally competent IPCs and represent a potential cell-based treatment for diabetes mellitus.
Similar content being viewed by others
Introduction
The skin maintains homoeostasis of cell proliferation, differentiation and regeneration through stem cells residing in the epidermis, dermis and appendages. Multipotent skin-derived precursors (SKPs) were recently isolated and expanded from human and other types of mammalian skin. These cells can differentiate into both neural and mesodermal progeny, including cell types rarely found in skin, such as neurons (Toma et al., 2001, 2005; Joannides et al., 2004). Thus, these cells may be useful for the replacement of not just skin tissue, but other tissues as well. SKPs are more easily obtained than other stem cells, which potentially makes them a useful autologous donor source for stem cell therapy. Although the multipotency of SKPs is well documented, research focusing on the differentiation and characterization of functional IPCs derived from SKPs is still in the early stages. Recently, ESCs have been shown to be able to differentiate into pancreatic islet-like clusters and to form pancreatic β cells. Lumelsky et al. (2001) designed a 5-stage protocol to induce ESCs to differentiate into IPCs. Hori et al. (2002) and Blyszczuk et al. (2003) refined Lumelsky's 5-stage induction protocol by either adding the growth inhibitor LY294002, or overexpressing the pax gene, respectively. In this study, we investigated whether human labia minora dermis-derived fibroblasts (hLMDFs) can differentiate into functional IPCs for pancreatic β cell replacement.
We isolated MSCs with characteristics of mesodermal progeny from hLMDFs. We describe the 3-stage differentiation protocol used to create IPCs directly from hLMDFs and provide evidence that hLMDF-derived functional IPCs transplanted under the kidney capsule can fully rescue streptozotocin-induced diabetic mice. This study strongly suggests that hLMDF-derived IPCs can be used clinically in the near future to treat patients with type 1 diabetes mellitus.
Results
Schematic representation of the 3-stage differentiation protocol
Differentiation of ES cells into IPCs has recently been achieved by bypassing EB formation and selectively generating IPCs (D'Amour et al., 2006). In this study, we designed a 3-stage differentiation protocol to assess the potential of hLMDFs to differentiate into IPCs, which is outlined in Figure 1.
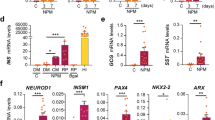
Outline of the 3-stage differentiation protocol and stage-specific cell cluster morphology. Schematic representation of the 3-stage protocol to derive insulin-producing cells (IPCs) from hLMDFs. Stage 1: Expansion of hLMDFs. hLMDFs were isolated from labia minora dermis and placed in growth medium. Stage 2: MSC-endoderm transition stage. Cells were cultured in serum-free DMEM/F12 media supplemented with ITS for 1 week. During this stage, the cells metamorphosed into round epithelial-like cells. After 7 days of stage 2, the cells were found to express nestin by FACS analysis and to express endoderm markers by RT-PCR and western blot analysis. Stage 3: Differentiation of pancreatic islet-like cell clusters. Cells were cultured in stage 3 medium (DMEM + 10% FBS + ITS mix + nicotinamide). The cells continued to differentiate and form larger clusters. Essential factor manipulations at each stage are also shown. Scale bars, 200 µm.
Isolation of adherent cells with fibroblast morphology from hLMDFs (Stage 1: MSC expansion)
Since SKP cells are readily available and have been shown to have osteogenic, chondrogenic, and adipogenic differentiation potential (Moon et al., 2008), we tested whether hLMDFs can serve as a source of SKP-type mesenchymal stem cells. hLMDFs were isolated and these cells formed an adherent monolayer within 4-5 days. hLMDFs showed a typical morphology of fibroblasts (stage 1: MSC expansion) (Supplemental Data Figure S1A) and cells from all 3 patients had a similar appearance (Supplemental Data Figure S1A). We routinely maintain cells in culture for 17-25 passages in our laboratory, and hLMDFs at passage 17 could be differentiated into IPCs (Supplemental Data Figure S1B), which indicates that these cells maintain their IPC differentiation potential in long-term culture. In addition, G-banding analysis of hLMDFs at passage 17 revealed a normal chromosomal karyotype (Supplemental Data Figure S1D), which indicates that these cells remain chromosomally stable over long-term culture. FACS and RT-PCR demonstrated that over 90% of hLMDFs expressed the typical MSC marker proteins (Supplemental Data Figure S1E and S1F), suggesting that hLMDFs exhibit MSC characteristics. To determine the possibility of these hLMDFs from hair follicle, we investigated the expression of SHOX, which is transcription factor for the development of hair follicles. We could detect expression of SHOX by RT-PCR, suggesting that we could not exclude the possible origin of hLMDFs from hair follicle (Supplemental Data Figure S1F). Furthermore, hLMDFs were able to differentiate into adipocytes, osteoblasts, chondrocytes, and smooth muscle cell types both in vitro and in vivo (Supplemental Data Figure S2). We found that hLMDFs from all 3 patients could be differentiated with similar efficiency into all 3 cell types (data not shown). Details of the results are provided as supplementary data.
Differentiation of hLMDFs into endoderm-like cells (Stage 2: MSC-endoderm transition)
To promote the differentiation of hLMDFs into endoderm-like cells, we first determined whether ITS could induce the expression of nestin and endoderm markers in hLMDFs. To this end, hLMDFs were cultured in serum-free DMEM/F12 supplemented with ITS for one week. During this stage, the rate of cell proliferation slowed and the spindle-like cells shortened and changed into round epithelial-like cells by the end of stage 2 (Figure 2A). We found that stage 2 differentiated cells expressed both nestin (Figure 2B) and Sox 17 (a marker of definitive and extraembryonic endoderm); Cer1, and CXCR4 (a marker of definitive endoderm); GATA4 (a marker of definitive and visceral endoderm); PDX1 (a marker of pancreatic endoderm); and Foxa2, on days 3 through 7 using RT-PCR, immunostaining and western blot analysis (Figures 2C-2E). PDX1 expression is involved in pancreas development, as both the exocrine and endocrine components of the pancreas develop from PDX1-expressing cells (Guz et al., 1995); PDX1 was also detected in the nuclei of cells derived from hLMDFs after completion of stage 2 (Figure 2D).
Generation of nestin-positive and endoderm-like cells from hLMDFs (stage 2). (A) The rate of cell proliferation was decreased during stage 2 treatment (upper panel), and cell morphology changed, resulting in round epithelial-like cells at this stage (lower panel). *P < 0.05 compared to stage 1. (B) Expression of nestin in induced hLMDFs was analyzed by FACS. Induced stage 1 and stage 2 hLMDFs were subjected to FACS analysis. Induced hLMDFs were nestin-positive after completion of stage 2. (C) RT-PCR analysis of endodermal marker genes during stage 2. Cells showed increased expression of nestin and endodermal-related genes during differentiation in stage 2. (D) Immunocytochemical analysis of pdx1 and GATA4 in stage 2. pdx1 expression was detected in stage 2. Nuclei were stained blue with DAPI. (E) Western blot analysis of Sox17, GATA4, and Pdx1 in stage 2. Expression of Sox17, GATA4, and Pdx1 was detected in stage 2.
Recent results have demonstrated that activin A can induce ESCs to differentiate into definitive endoderm cells (Kubo et al., 2004) and improve insulin secretion from cultured human pancreatic islets (Florio et al., 2000). Therefore, we tested whether activin A could enhance the differentiation of hLMDFs into endoderm-like cells in this study. Treatment of hLMDFs with both ITS and activin A (ITS + AA) at stage 2 induced expression of endoderm markers (Supplemental Data Figures S3A and S3B). Addition of activin A alone, however, suppressed both the growth of hLMDFs (Supplemental Data Figure S3C) and differentiation into insulin-producing cells (data not shown).
We also tested the effects of several ECM molecules, including matrigel, fibronectin, and gelatin, on the proliferation of hLMDFs in our differentiation protocols. We found that treatment with ECM molecules did not significantly enhance the proliferation of hLMDFs (Supplemental Data Figures S3C and S4A-S4C). Therefore, we found that ITS alone, without an ECM, can facilitate the development of endoderm-like cells.
Dynamics of pancreatic gene expression during the differentiation of hLMDFs into IPCs (stage 3: differentiation into pancreatic islet-like clusters)
To further promote the differentiation of hLMDFs into IPCs, the differentiated cells from stage 2 were shifted into culture medium containing low-glucose DMEM supplemented with ITS and nicotinamide, which promote the maturation of pancreatic β cells (Lumelsky et al., 2001). These cells continued to differentiate and formed larger clusters (stage 3: IPCs) (Figure 3A). To detect whether endoderm- and pancreatic-specific genes were expressed in the differentiated cells, RT-PCR and western blotting analysis were performed at each stage of the differentiation process (Figures 3B and 3C). The epithelial-like cells induced by ITS expressed definitive endoderm-specific genes, such as Foxa2, GATA4, Sox 17, CXCR4, CER, Ngn 3, and Mixl1 (Kroon et al., 2008; Raikwar and Zavazava, 2009; Ji et al., 2010), at high levels after completion of stage 2, but not after stage 1. After end of stage 3, the differentiated cells strongly expressed endocrine- and pancreas-specific genes, including insulin, GK, PP, NKX6.1, Kir6.2, PC1/3 and PAX6. In addition, cells in the islet-like clusters, but not undifferentiated cells, were distinctly stained crimson red by DTZ (Figure 3E). Expression of PDX1, c-peptide, and insulin, but not glucagon or PP, was clearly detected in most differentiated cells at stage 3 (Figures 3C and 3D). C-peptide positive cells accounted for 50% of differentiated cells. Furthermore, we found that successful differentiation into IPCs was detected only when cells went through the sequential differentiation process from stages 1 to 2 to 3. If cells went directly from stage 1 to stage 3, there was a complete lack of differentiation into IPCs, and hLMDFs underwent cell death (Supplemental Data Figure S5). To investigate whether insulin secretion from hLMDF-derived IPCs could be regulated by glucose, cells were treated with Krebs-Ringer buffer containing either a low (3 Mm) or high (16.7 Mm) concentration of glucose, and then insulin was measured in the culture medium by ELISA (Chandra et al., 2009). Differentiated IPCs (stage 3), but not undifferentiated hLMDFs (stage 1), secreted insulin in a glucose-dependent manner, and insulin release in the high-glucose medium was nearly 7 times higher than that in the low-glucose medium (Figure 3F). In addition, the direct depolarization of the induced IPCs in response to the addition of KCl to the media resulted in prominent increases in secreted insulin within 30 min (Figure 3G). These results indicate that hLMDFs can be induced to differentiate into functional IPCs, suggesting that they have secretory characteristics similar to those of pancreatic β cells (Kroon et al., 2008).
Differentiation of hLMDFs into insulin-producing cells (stage 3). (A) hLMDFs were cultured in DMEM/F12 medium with ITS mix for 7 days (stage 2: upper panel) and then switched to DMEM (low glucose) supplemented with 10% FBS, ITS mix, and nicotinamide for 7 days (stage 3: lower panel). The cells continued to differentiate and eventually formed larger clusters after completion of stage 3. These clusters were collections of differentiated cells that were intensely stained by DTZ. (B) RT-PCR analysis of endodermal and pancreatic cell-related genes in induced hLMDFs. These cells expressed endodermal-related genes in stage 2, and hLMDF-derived IPCs (stage 3) expressed pancreatic cell-related genes. Capan-2 was used as a control. (C) Western blot analysis of endodermal and pancreatic cell-related genes in induced hLMDFs. (D) hLMDF-derived IPCs stained crimson red by DTZ are visible, with some cells assembled in groups. (E) Immunocytochemical analysis of Pdx1, insulin, and c-peptide in stage 3. Nuclei were stained blue with DAPI. Expression of both insulin and c-peptide was detected in most stage 3 IPCs. All experiments were repeated at least three times. (F) hLMDF-derived IPCs (stage 3) exhibit glucose-stimulated insulin secretion. hLMDF-derived IPCs were sequentially treated with low (3 mM) and high (16.7 mM) concentrations of glucose and the supernatants were collected and analyzed for insulin content by ELISA. *P < 0.05 compared to 3 mM glucose. (G) After induction for 7 days in stage 3, hLMDF-derived IPCs secreted insulin in response to KCl. *P < 0.05 compared to vehicle treatment.
Transplantation of insulin-producing cells rescues glucose homeostasis in diabetic animals
To investigate whether IPCs could rescue mice with diabetes, we transplanted the differentiated cells into diabetic nude mice. Cells were transplanted under the kidney capsules of hyperglycemic mice after their blood glucose levels reached ≥250 mg/dl, and glucose levels were then monitored for 5 weeks. Cells from each of the 3 differentiation stages were transplanted into mice (n = 4 per group). Non-transplanted diabetic mice remained hyperglycemic with serum glucose levels of approximately 280-300 mg/dl until death. Similarly, transplanted mice that received low glucose DMEM-cultured cells (stage 1) or ITS-treated cells (stage 2) did not undergo rescue of glucose levels and died with hyperglycemia. In contrast, transplanted mice that received hLMDF-derived IPCs (stage 3) exhibited normalization of their blood glucose levels (Figure 4A). To exclude the possibility of a heterogeneous cell population in this study, and to assess the functional constancy of transplanted IPCs in vivo, hLMDFs were infected with a retrovirus encoding EGFP and 3 clones of EGFP-labeled hLMDFs (EGFP-hLMDFs) were obtained by single-cell cloning (Supplemental Data Figure S6). EGFP-hLMDF-derived IPCs were transplanted under the kidney capsules of STZ-treated diabetic mice as described above. The blood glucose of the induced cell-transplanted mice (clones 1, 2 and 3; n = 15) was maintained at normal levels for nearly 33 days (Figure 4B). To further evaluate the function of the implanted EGFP-hLMDF-derived IPCs, we performed intraperitoneal glucose tolerance tests on induced IPC-implanted mice and non-diabetic control mice 33 days after transplantation. As shown in Figure 4C, blood glucose levels in the non-diabetic control mice rose rapidly, with peak values obtained at 2 min, followed by a return to the normal range between 6 and 9 min. Similarly, blood glucose levels in the EGFP-hLMDF-derived IPC-implanted mice were generally higher, but likewise displayed a peak at 2 min, followed by a return to the normal range between 6 and 9 min. Furthermore, the transplanted cells showed expression of PDX1, c-peptide, and EGFP 33 days after transplantation. But another pancreas-specific marker, glucagon, was not detected by immunostaining (Figure 4D). These results clearly demonstrate that hLMDF-derived IPCs improve glucose control and functionally rescue STZ-induced diabetic mice.
Remission of diabetes in response to the transplantation of stage 3 hLMDF-derived IPCs into diabetic mice. Induced hLMDFs (stage 1-stage 3) were transplanted under the kidney capsules of streptozotocin-induced diabetic mice (blood glucose levels ≥250 mg/dl). (A) Glucose levels at the indicated time points after transplantation are given as means ± SDs. (B) Induced EGFP-hLMDF-derived IPCs (stage 3) were transplanted under the kidney capsule of streptozotocin-induced diabetic mice and blood glucose levels were monitored. (C) Intraperitoneal glucose tests were performed 28 d after transplantation. (D) 33 days after transplantation, the kidneys were removed and sectioned. EGFP-hLMDF-derived IPCs were present in the kidney capsule and were immunopositive for Pdx1, and c-peptide. However, they were immunonegative for glucagon. Tissue sections were stained with the indicated antibodies. Nuclei were stained blue with DAPI. Scale bars, 50 µm.
Discussion
Recent studies have suggested the possibility of generating IPCs from pancreas (Bonner-Weir et al., 2000; Ramiya et al., 2000), intestinal epithelium (Suzuki et al., 2003), human (Assady et al., 2001) and mouse (Lumelsky et al., 2001) embryonic stem cells. Despite the great potential of this procedure and conceptual advances, some obstacles, such as immune rejection, have proved difficult to overcome. Skin-derived precursors (SKPs), which exhibit the differentiation potential for the ectodermal and mesodermal lineages, as well as germ cells (Toma et al., 2001, 2005; Joannides et al., 2004; Dyce et al., 2006), have been known for years to represent a safe and abundant source of large quantities of adult stem cells (Toma et al., 2001, 2005; Joannides et al., 2004). Furthermore, previous studies have found that skin-derived stem cells share many features with pancreatic stem cells (Kajahn et al., 2008) and can differentiate into IPCs (Guo et al., 2009), but fail to rescue glucose homeostasis when transplanted into diabetic animals (Guo et al., 2009). To this end, we explored the possibility of inducing hLMDFs to differentiate into IPCs under specific in vitro culture conditions.
Our present results demonstrate that functional IPCs can be generated from hLMDFs using a 3-stage differentiation protocol. In stage 1, hLMDFs exhibited phenotypic characteristics similar to those of MSCs derived from other sources, such as term umbilical cord blood and bone marrow (In 't Anker et al., 2003; Cha and Falanga, 2007). In stage 2, we explored previous findings showing similarities between the development of β cells and of neuroepithelial cells (Edlund, 2001), and similar transient expression of nestin was reported to occur in human insulin-producing β cell precursors (Zulewski et al., 2001; Blyszczuk et al., 2003). To effectively differentiate hLMDFs into nestin-positive cells, we cultured hLMDFs in serum-free ITS conditions, which induced a round, epithelial-like cell morphology with the concurrent appearance of nestin- and definitive endoderm-positive cells. (Lumelsky et al., 2001). Furthermore, we found that successful differentiation into functional pancreatic β cell was detected only when cells were cultured in ITS-medium without serum supplement. In the presence of serum in stage 2, we could not induce morphological change of hLMDFs into epithelial-like cells and endodermal marker gene expressions.
Previous studies have shown that nicotinamide can induce the differentiation and maturation of human fetal pancreatic islet cells (Otonkoski et al., 1993). Also, duct tissues treated with nicotinamide can be made to differentiate into glucose-responsive islet tissue in vitro (Bonner-Weir et al., 2000). Furthermore, nicotinamide promotes in vitro transdifferentiation and maturation of liver stem cells into IPCs (Yang et al., 2002). Thus, at the end of the differentiation procedure (stage 3), nicotinamide was added to the media to promote differentiation and maturation of precursor cells into IPCs. Indeed, we found that nicotinamide promoted the in vitro differentiation and maturation of stage 2 hLMDFs, but not stage 1 hLMDFs, into IPCs. Taken together, we found that the combination of ITS and nicotinamide effectively promoted the differentiation of hLMDFs into IPCs. This study shows that IPCs produced through our 3-stage differentiation protocol have many similarities to mature islet β cells in vitro and in vivo. First, IPCs obtained through this approach express most of the crucial β cell transcription factors and functional markers, including Pdx1, Nkx6.1, insulin, Kir6.2, and PC1/3. The expression of insulin, Pdx1 and Nkx6.1 is considered to be a specific functional characteristic of mature β cells and has previously been reported only in islet β cells in vivo (Murtaugh, 2007). Second, IPCs secrete insulin in response to stimulation with glucose or KCl, suggesting that these cells possess the major functional capabilities of β cells, namely insulin release in response to changes in extracellular glucose concentrations and the presence of insulin-containing secretory granules (Easom, 2000). Third, induced homogenous IPCs have the ability to functionally replace β cells in vivo. After implantation of induced IPCs, the blood glucose levels of diabetic mice decreased to normal levels and remained normal for 37 days while their body weights continued to increase. Furthermore, an intraperitoneal glucose tolerance test showed that the blood glucose levels of transplanted diabetic mice exhibited similar kinetics to those of normal control mice (Tang et al., 2004), representing a recovery of insulin secretory ability in the IPC-transplanted mice. Our findings also show that EGFP-labeled insulin- and c-peptide-positive homogeneous IPCs survived for more than 30 days after transplantation. Taken together, these results suggest that these induced cells have a similar function to β cells both in vitro and in vivo.
In conclusion, we have successfully differentiated hLMDFs into functional IPCs with the ability to produce insulin. Therefore, our findings present evidence that skin-derived MSCs can be induced to differentiate into functional IPCs and may provide a source of β cells for the treatment of type 1 diabetes. Furthermore, hLMDFs are an excellent source of stem cells for conversion into functional IPCs due to their rapid availability and the low risk of ethical concerns.
Methods
Preparation of human labia minora dermis-derived fibroblasts (hLMDFs)
With patient consent, human skin from the labia minora dermis was obtained from patients (45, 47, and 48-year-old women) who underwent cosmetic surgery, and collected in Hanks' balanced salt solution (HBSS) (Invitrogen, Carlsbad, CA) at 4℃. All experimental protocols in this study were conducted with strict adherence to the guidelines of the Institutional Review Board of Korea University. 1-2 cm2 pieces of human skin were washed with HBSS, cut into 4- to 6-mm pieces, washed again, and incubated in Dispase (Invitrogen) overnight at 4℃ as previously described (Chen et al., 2007; Moon et al., 2008). Briefly, the epidermis was manually removed from each tissue piece, and the dermis was cut into small pieces and incubated in Collagenase type IV (Invitrogen) for 30-60 min at 37℃. Then samples were centrifuged at 1,000 rpm for 5 min. Following removal of the supernatant fraction, the precipitate was washed with growth medium (GM: Low-glucose DMEM + 10% FBS + 1% L-glutamine + 1% penicillin/streptomycin) and centrifuged at 1,000 rpm for 5 min. The precipitate was resuspended in the remaining medium using a fire-polished Pasteur pipette, and the suspension was passed through a 70-µm cell strainer (BD Bioscience, Mississauga, Ontario, Canada). The strained cell suspension was centrifuged and resuspended with GM. Harvested cells were seeded at a density of 1 ×105 cells/cm2 on a 10-cm2 tissue culture plate (BD Bioscience) and incubated at 37℃ with 5% CO2 (Chen et al., 2007; Moon et al., 2008). The cells were passaged by removing the medium and incubating the cells with 0.05% trypsin/EDTA containing 1 mM EDTA for 3 min at 37℃. The cells were passaged every 3 days. The cells were continuously passaged and cell doubling times were calculated. To determine the lifespan of hLMDFs, primary passage 2 cells were plated at a density of 3 ×105 cells per 10 cm tissue culture plate and passaged every 3 days following the standard 3T3 protocol with the number of population doublings calculated per day (You et al., 2004). Capan-2 cells, which comprise a human pancreatic adenocarcinoma cell line, were purchased from the American Type Culture Collection (ATCC) and cultured as previously described (Lowe et al., 2007).
Differentiation of pancreatic β cells from hLMDFs
Differentiation of hLMDFs into pancreatic β cells was performed in 3 stages. In stage 1, hLMDFs were cultured in GM. In stage 2, hLMDFs were cultured in DMEM/F12 medium (Invitrogen) with ITS mix (insulin, transferrin, and selenium, Sigma) for 7 days (Lumelsky et al., 2001). In stage 3, cells were cultured in low glucose DMEM supplemented with 10% FBS, 100 units/ml penicillin/streptomycin, ITS mix, and 10 mM nicotinamide (Sigma-Aldrich) for 7 days (Zalzman et al., 2005).
Karyotyping analysis
Karyotyping was performed by the Cytogenomic Services Facility of Samkwang Medical Laboratories as previously described (Yoo et al., 2005). At least 100 metaphase cells were analyzed, and a minimum of 10 were karyotyped for each line.
FACS analysis
Cells were trypsinized, centrifuged, and 500,000 cells were resuspended in 100 µl of wash buffer (PBS containing 10% serum). After rinsing twice with cold buffer (DPBS containing 1% bovine serum albumin (BSA, Sigma)), cells were incubated with primary antibody (Supplemental Data Table S2) at 4℃ for 1 h, washed twice with 1% BSA in PBS, resuspended in 100 µl of fluorescein isothiocyanate (FITC)-labeled secondary antibody (diluted 1:100 in PBS with 1% BSA), and incubated for an additional 40 min at 4℃ for FACS analysis (Yoon et al., 2010). All FACS analysis experiments were repeated at least three times.
RT-PCR
Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's protocol. cDNA was generated using reverse transcriptase II (Invitrogen) according to the manufacturer's instructions. Twenty-five nanograms of cDNA were combined with PCR primers for various marker genes (Bioneer, Daejeon) under the conditions outlined in Supplemental Data Table S1. All PCR products in this study were tested to ensure that the number of PCR cycles was in the linear range. These values were analyzed and normalized as previously described (Kho et al., 2008).
Adipogenic, osteogenic and chondrogenic differentiation
Adiopogenic, osteogenic and chondrogenic differentiation of each sample was quantitated according to previously described protocols (Kern et al., 2006; Moon et al., 2008; Yoon et al., 2010).
Smooth muscle cell differentiation
Differentiation of each sample was induced using a previously described protocol (Ross et al., 2006; Moon et al., 2008). Briefly, cells were induced to differentiate into smooth muscle-like cells by cultivation on 4-well plates (Nalgen, Nunc) for 6 days in serum-free MAPC medium [60% DMEM-low glucose (Invitrogen), 40% MCDB-201 (Sigma-Aldrich) containing 1× insulin-transferrin-selenium, 1× linoleic acid BSA, 10-9 M dexamethasone (Sigma-Aldrich), 10-4 M ascorbic acid 2-phosphate (Sigma-Aldrich), and 1% penicillin-streptomycin (Cambrex)], supplemented with 2.5 ng/ml TGF-β1 (R&D) and 5 ng/ml PDGF-BB (R&D) as described previously.
Immunocytochemistry
Immunostaining was carried out using a previously described protocol (Moon et al., 2008; Yoon et al., 2010).
Western blot analysis
Cells were resuspended in ice-cold RIPA lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Nonidet-40 (NP-40), 0.5% sodium deoxycholate, 1% SDS, 1 mM sodium orthovanadate, 1 mM b-glycerol phosphate, 1 mM sodium fluoride, and 2.5 mM sodium pyrophosphate) with a protease inhibitor cocktail (complete mini tablet, Roche, Penzberg, Germany). Samples were incubated on ice for 30 min and supernatants were recovered by centrifugation at 14000 rpm for 30 min at 4℃. Aliquots of 50-100 µg of protein per lane were separated on a 4-12% gradient or 10% SDS-PAGE NuPAGE gels (Invitrogen) and transferred to PVDF membranes (Millipore, Roma, Italy). The membranes were blocked in Tris-buffered saline with 0.1% tween-20 and 3% milk, and incubated with primary antibodies (Supplemental Data Table S2). Membranes were then incubated with horseradish peroxidase-conjugated anti-secondary IgG (Invitrogen) antibody and visualized using the Super Signal West Pico Chemiluminescent Substrate (Pierce, IL).
Dithizone (DTZ) staining
In vitro DTZ staining was performed by adding 10 µl of the stock solution to 1 ml of culture medium without insulin. Culture dishes were incubated at 37℃ for 15-30 min. After the dishes were rinsed 3 times with PBS, red-stained clusters were examined using a phase contrast microscope and the number of DTZ-stained cells in each culture was determined (Shiroi et al., 2002).
Insulin secretion assay
Differentiated cells were washed 3 times with KRB buffer (120 mM NaCl2, 5 mM KCl, 2.5 mM CaCl2, 10 mM HEPES, 1.1 mM NaHCO3, and 0.5% bovine serum albumin), and then pre-incubated in KRBH buffer containing 5.6 mM glucose for 1 h, followed by the removal of the buffer and incubation with 1 ml KRBH buffer containing 3 mM or 16.7 mM glucose and 10 µM KCl for 30 min. The culture supernatants were collected for measurement of insulin using a human insulin ELISA kit (Linco) (Chandra et al., 2009). All values were determined using a standard curve for human insulin.
Generation of EGFP-labeled single cell clone-derived hLMDFs
To produce amphotropic retrovirus, pBabe-EGFP plasmid DNA was transfected into a PT67 packaging cell line with LipofectAMINE™ 2000 (Invitrogen) and then several stable retrovirus-producing PT67 EGFP cell lines were acquired using antibiotic selection. For infection, three hLMDFs were incubated for approximately 20 h with the conditioned medium collected from the retrovirus-producing cell line PT67/EGFP and 6 µg/ml polybrene (Sigma-Aldrich). For EGFP gene transduction, 70% confluent hLMDFs were incubated with the retroviral supernatants of PT67 EGFP cells containing 10% FBS for 24 h. The medium was replenished at this time. Cells were harvested with trypsin/EDTA 24 h post-transduction and subcultured at a ratio of 1:3 into selective medium, which contained 0.5 µg/ml puromycin. hLMDF clones expressing high levels of EGFP (EGFP-hLMDFs) were isolated by cloning using trypsin/EDTA and amplified using conventional culture methods (Yamamoto et al., 2003; Zhang et al., 2005).
Transplantation
Six week-old Balb/c-nu female mice (Central Lab., Animal Inc., Korea) were administered a single intraperitoneal injection of streptozotocin (STZ: Sigma-Aldrich; 200 mg/kg of body weight). On the day that their blood glucose levels reached >250 mg/dl, mice were either engrafted with 2 ×106 cells differentiated in culture, or received a sham transplant (20 µl of culture medium) in the capsule of the right kidney using a 26-gauge needle. Blood glucose levels were measured every 3 days in samples obtained from the tail vein using a blood glucose test meter and strips (Super Glucocard II, Donbang Int., Korea). Body weight was monitored every 2-4 days. Grafts were removed 4 weeks after transplantation and were analyzed by immunohistochemistry and hematoxylin/eosin staining (Zalzman et al., 2005; Shim et al., 2007). For the intraperitoneal glucose tolerance test (IPTGG), non-transplanted diabetic mice and diabetic mice with normalized glucose levels following cell transplantation were fasted for 10 min and then given an i.p. injection of glucose. Blood glucose was monitored every 10 min after glucose injection (Li et al., 2007).
Histological analysis
Kidney tissue was fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned. Sections were rehydrated, washed in PBS, and unmasked with unmasking solution according to the manufacturer's instructions. Sections were blocked for 1 h in 0.3% triton X-100 with 5% serum, incubated overnight at 4℃ with primary antibodies (Supplemental Data Table S2), and then incubated for 1 h at room temperature with secondary antibodies, stained with DAPI, and mounted on slides.
Statistical analysis
All values are expressed as means ± SD. Student's paired t-test was performed for comparisons of paired samples. A probability (P) value < 0.01 or 0.05 was considered to be significant.
Abbreviations
- hLMDFs:
-
human labia minora dermis-derived fibroblasts
- IPCs:
-
insulin-producing cells
- PDX1:
-
pancreatic and duodenal homeobox 1
- STZ:
-
streptozotocin
References
Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M . Insulin production by human embryonic stem cells . Diabetes 2001 ; 50 : 1691 - 1697
Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM . Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells . Proc Natl Acad Sci USA 2003 ; 100 : 998 - 1003
Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ . In vitro cultivation of human islets from expanded ductal tissue . Proc Natl Acad Sci USA 2000 ; 97 : 7999 - 8004
Cha J, Falanga V . Stem cells in cutaneous wound healing . Clin Dermatol 2007 ; 25 : 73 - 78
Chandra V, G S, Phadnis S, Nair PD, Bhonde RR . Generation of pancreatic hormone-expressing islet-like cell aggregates from murine adipose tissue-derived stem cells . Stem Cells 2009 ; 27 : 1941 - 1953
Chen FG, Zhang WJ, Bi D, Liu W, Wei X, Chen FF, Zhu L, Cui L, Cao Y . Clonal analysis of nestin(-) vimentin(+) multipotent fibroblasts isolated from human dermis . J Cell Sci 2007 ; 120 : 2875 - 2883
D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE . Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells . Nat Biotechnol 2006 ; 24 : 1392 - 1401
Dyce PW, Wen L, Li J . In vitro germline potential of stem cells derived from fetal porcine skin . Nat Cell Biol 2006 ; 8 : 384 - 390
Easom RA . Beta-granule transport and exocytosis . Semin Cell Dev Biol 2000 ; 11 : 253 - 266
Edlund H . Developmental biology of the pancreas . Diabetes 2001 ; 50 : S5 - S9
Florio P, Luisi S, Marchetti P, Lupi R, Cobellis L, Falaschi C, Sugino H, Navalesi R, Genazzani AR, Petraglia F . Activin A stimulates insulin secretion in cultured human pancreatic islets . J Endocrinol Invest 2000 ; 23 : 231 - 234
Guo W, Miao C, Liu S, Qiu Z, Li J, Duan E . Efficient differentiation of insulin-producing cells from skin-derived stem cells . Cell Prolif 2009 ; 42 : 49 - 62
Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G . Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny . Development 1995 ; 121 : 11 - 18
Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK . Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells . Proc Natl Acad Sci USA 2002 ; 99 : 16105 - 16110
In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH . Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation . Blood 2003 ; 102 : 1548 - 1549
Ji AR, Ku SY, Cho MS, Kim YY, Kim YJ, Oh SK, Kim SH, Moon SY, Choi YM . Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage . Exp Mol Med 2010 ; 42 : 175 - 186
Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S . Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells . Lancet 2004 ; 364 : 172 - 178
Kajahn J, Gorjup E, Tiede S, von Briesen H, Paus R, Kruse C, Danner S . Skin-derived human adult stem cells surprisingly share many features with human pancreatic stem cells . Eur J Cell Biol 2008 ; 87 : 39 - 46
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K . Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue . Stem Cells 2006 ; 24 : 1294 - 1301
Kho Y, Kim S, Yoon BS, Moon JH, Kim B, Kwak S, Woo J, Oh S, Hong K, Kim H, You S, Choi Y . Induction of serum amyloid A genes is associated with growth and apoptosis of HC11 mammary epithelial cells . Biosci Biotechnol Biochem 2008 ; 72 : 70 - 81
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE . Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo . Nat Biotechnol 2008 ; 26 : 443 - 452
Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G . Development of definitive endoderm from embryonic stem cells in culture . Development 2004 ; 131 : 1651 - 1662
Li Y, Zhang R, Qiao H, Zhang H, Wang Y, Yuan H, Liu Q, Liu D, Chen L, Pei X . Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells . J Cell Physiol 2007 ; 211 : 36 - 44
Lowe AW, Olsen M, Hao Y, Lee SP, Taek Lee K, Chen X, van de Rijn M, Brown PO . Gene expression patterns in pancreatic tumors, cells and tissues . PLoS One 2007 ; 2 : e323 -
Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R . Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets . Science 2001 ; 292 : 1389 - 1394
Moon JH, Kwak SS, Park G, Jung HY, Yoon BS, Park J, Ryu KS, Choi SC, Maeng I, Kim B, Jun EK, Kim S, Kim A, Oh S, Kim H, Kim KD, You S . Isolation and characterization of multipotent human keloid-derived mesenchymal-like stem cells . Stem Cells Dev 2008 ; 17 : 713 - 724
Murtaugh LC . Pancreas and beta-cell development: from the actual to the possible . Development 2007 ; 134 : 427 - 438
Otonkoski T, Beattie GM, Mally MI, Ricordi C, Hayek A . Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells . J Clin Invest 1993 ; 92 : 1459 - 1466
Raikwar SP, Zavazava N . Insulin producing cells derived from embryonic stem cells: are we there yet ? J Cell Physiol 2009 ; 218 : 256 - 263
Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG . Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells . Nat Med 2000 ; 6 : 278 - 282
Ross JJ, Hong Z, Willenbring B, Zeng L, Isenberg B, Lee EH, Reyes M, Keirstead SA, Weir EK, Tranquillo RT, Verfaillie CM . Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells . J Clin Invest 2006 ; 116 : 3139 - 3149
Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R, Kim JH . Directed differentiation of human embryonic stem cells towards a pancreatic cell fate . Diabetologia 2007 ; 50 : 1228 - 1238
Shiroi A, Yoshikawa M, Yokota H, Fukui H, Ishizaka S, Tatsumi K, Takahashi Y . Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone . Stem Cells 2002 ; 20 : 284 - 292
Suzuki A, Nakauchi H, Taniguchi H . Glucagon-like peptide 1 (1-37) converts intestinal epithelial cells into insulin-producing cells . Proc Natl Acad Sci USA 2003 ; 100 : 5034 - 5039
Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ . In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow . Diabetes 2004 ; 53 : 1721 - 1732
Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD . Isolation of multipotent adult stem cells from the dermis of mammalian skin . Nat Cell Biol 2001 ; 3 : 778 - 784
Toma JG, McKenzie IA, Bagli D, Miller FD . Isolation and characterization of multipotent skin-derived precursors from human skin . Stem Cells 2005 ; 23 : 727 - 737
Yamamoto N, Yang M, Jiang P, Xu M, Tsuchiya H, Tomita K, Moossa AR, Hoffman RM . Real-time imaging of individual fluorescent-protein color-coded metastatic colonies in vivo . Clin Exp Metastasis 2003 ; 20 : 633 - 638
Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB . In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells . Proc Natl Acad Sci USA 2002 ; 99 : 8078 - 8083
Yoo SJ, Yoon BS, Kim JM, Song JM, Roh S, You S, Yoon HS . Efficient culture system for human embryonic stem cells using autologous human embryonic stem cell-derived feeder cells . Exp Mol Med 2005 ; 37 : 399 - 407
Yoon BS, Moon JH, Jun EK, Kim J, Maeng I, Kim JS, Lee JH, Baik CS, Kim A, Cho KS, Lee HH, Whang KY, You S . Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells . Stem Cells Dev 2010 ; 19 : 887 - 902
You S, Heo M, Moon JH, Kim SC, Kwak S, Yoon DH, Jin D, Hong KC, Foster DN, Choi YJ, Kim H . Establishment of life-span extended bovine fibroblast cells carrying the characterization of primary cells . Mol Cells 2004 ; 18 : 261 - 268
Zalzman M, Anker-Kitai L, Efrat S . Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype . Diabetes 2005 ; 54 : 2568 - 2575
Zhang H, Zhao Y, Zhao C, Yu S, Duan D, Xu Q . Long-term expansion of human neural progenitor cells by epigenetic stimulation in vitro . Neurosci Res 2005 ; 51 : 157 - 165
Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, Thomas MK, Habener JF . Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine,exocrine, and hepatic phenotypes . Diabetes 2001 ; 50 : 521 - 533
Acknowledgements
This research was supported by a grant (SC-5150) from the Stem Cell Research Center of the 21st Century Frontier Research Program, funded by the Ministry of Education, Science and Technology, Republic of Korea; a grant (08172KFDA527) from the Korea Food and Drug Administration, Republic of Korea; a National Research Foundation (NRF) grant (No. 2010-0020347) funded by the Korea Government (MEST).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kim, B., Yoon, B., Moon, JH. et al. Differentiation of human labia minora dermis-derived fibroblasts into insulin-producing cells. Exp Mol Med 44, 26–35 (2012). https://doi.org/10.3858/emm.2012.44.1.002
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2012.44.1.002
Keywords
This article is cited by
-
Transplantation of human dental pulp stem cells in streptozotocin-induced diabetic rats
Anatomical Science International (2020)
-
Genome-wide association studies for the concentrations of insulin, triiodothyronine, and thyroxine in Chinese Holstein cattle
Tropical Animal Health and Production (2020)
-
Adult muscle-derived stem cells engraft and differentiate into insulin-expressing cells in pancreatic islets of diabetic mice
Stem Cell Research & Therapy (2017)
-
Cell therapy in diabetes: current progress and future prospects
Science Bulletin (2015)
-
Differentiation of human skin-derived precursor cells into functional islet-like insulin-producing cell clusters
In Vitro Cellular & Developmental Biology - Animal (2015)