Abstract
Indoleamine 2,3-dioxygenase (IDO) is a key negative regulator of immune responses and has been implicated in tumor tolerance, autoimmune disease and asthma. IDO was detected in the joint synovial tissue in the inflammatory microenvironment of rheumatoid arthritis (RA), but IDO expression in joint synovial tissue is not sufficient to overcome the inflamed synovial environment. This study aimed to unravel the mechanisms involving the failure to activate tolerogenic IDO in the inflamed joint. We demonstrate that both poly (I:C) and lipopolysaccharide (LPS) induce expression of IDO in synovial fibroblasts. However, inflammatory cytokines such as IL-17, TNF-α, IL-12, IL-23 and IL-16 did not induce IDO expression. Poly (I:C) appeared to induce higher IDO expression than did LPS. Surprisingly, toll-like receptor (TLR)4-mediated IDO expression was upregulated after depletion of myeloid differentiation primary response protein 88 (MyD88) in synovial fibroblasts using small interfering RNA (siRNA). IDO, TLR3 and TLR4 were highly expressed in synovial tissue of RA patients compared with that of osteoarthritis patients. In addition, RA patients with severe disease activity had higher levels of expression of IDO, TLR3 and TLR4 in the synovium than patients with mild disease activity. These data suggest that upregulation of IDO expression in synovial fibroblasts involves TLR3 and TLR4 activation by microbial constituents. We showed that the mechanisms responsible for IDO regulation primarily involve MyD88 signaling in synovial fibroblasts, as demonstrated by siRNA-mediated knockdown of MyD88.
Similar content being viewed by others
Introduction
The immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO) is the rate-limiting enzyme that depletes tryptophan in local tissue environments. Tryptophan deprivation and the production of proapoptotic kynurenines via IDO regulate T cell proliferation and survival in vitro and in vivo, which is important in tolerance induction (Mellor and Munn, 1999; Munn et al., 1999). IDO expression has been shown to be critical for allogeneic fetal tolerance, tumor tolerance and suppression of autoimmunity (Munn et al., 1998; Mellor and Munn, 2001; Munn et al., 2004). Furthermore, IDO controls T cell responses in autoimmune disorders and regulates the severity of a variety of experimental autoimmune disorders (Sakurai et al., 2002; Grohmann et al., 2003; Szanto et al., 2007). IDO protein is widely expressed in most tumor cells, dendritic cells, macrophages, microglia, eosinophils, fibroblasts and endothelial cells (Uyttenhove et al., 2003; Mellor and Munn, 2004; Munn and Mellor, 2004; Odemuyiwa et al., 2004; Beutelspacher et al., 2006). In immune cells, IDO expression is regulated and induced by various stimuli such as cytokines (e.g. IFN-γ and IFN-α), pathogen-associated molecular patterns (e.g. lipopolysaccharide (LPS), CpG oligodeoxynucleotides (CpG ODN) and other bacterial antigens) and costimulatory molecules (such as the cytotoxic T lymphocyte antigen 4-B7 interaction) (Munn, 2006).
Autoimmune disease arises when immunological tolerance breaks down. Rheumatoid arthritis (RA) is also associated with a breaking of self-tolerance to joint-specific antigens (Maffia et al., 2004). The main features of RA are chronic inflammation and hyperplasia of the synovial tissue as a result of an accumulation of macrophage-like and fibroblast-like synoviocytes (FLS) and infiltration by T and B cells, plasma cells and dendritic cells (Tak and Bresnihan, 2000; Cha et al., 2010). Although factors that contribute to the pathogenesis of RA are unknown, microbes and their products (microbial constituents) are one of the potential triggers associated with inflammatory and immune responses in RA. In the joints of patients with RA, exogenous and endogenous toll-like receptor (TLR) ligands, such as peptidoglycan and necrotic cells, have been identified. Rheumatoid synovial fibroblasts are resident cells of the synovial membrane that participate in the initiation and perpetuation of RA. Synovial fibroblasts activated via TLR ligation by endogenous TLR ligands in the synovial fluid of patients with RA contribute to the pathogenesis of inflammatory arthritis (Brentano et al., 2005). Although the interaction of TLRs with their microbial ligands activates innate immunity to mount a defense (Takeda et al., 2003), it also elicits a counterregulatory response. Previous studies demonstrated that bacterial DNA, its synthetic immunostimulatory sequence oligodeoxynucleotide (ISS-ODN) analogs (TLR9 ligands), LPS (TLR4 ligand) and immunostimulatory RNAs or R848 (TLR7/8 ligands) induce IDO expression in vivo and in vitro (Hissong et al., 1995; Hayashi et al., 2001; Furset et al., 2008). In particular, induction of IDO by CpG ODN, signaling through TLR9, has been described in splenic CD19+ dendritic cells (Mellor et al., 2005) or plasmacytoid dendritic cells (pDC). Human gingival fibroblasts are also known to express IDO when treated with ligands for TLR2, TLR3, TLR4 and TLR5 (Mahanonda et al., 2007). Although signaling through TLRs induces IDO expression, the mechanisms of the mutual relationship between TLR signaling and IDO induction have not been defined. Previous studies showed that exposure of synovial fibroblasts to IFN-γ caused a massive increase in IDO expression (Yuan et al., 1998). Moreover, IDO was activated in the synovial membrane of RA (Igari et al., 1987) and the decrease of tryptophan in serum of patients with RA correlated well with greater disease activity (Schroecksnadel et al., 2006). These findings suggest that certain factors in the inflammatory milieu of the joints in RA may induce high levels of IDO expression. We hypothesized that ligands for TLRs present in the inflamed joint could induce and regulate IDO expression in synovial fibroblasts. Our results demonstrate that the myeloid differentiation primary response gene 88 (MyD88) pathway negatively regulates IDO expression in synovial fibroblasts.
Results
IDO expression in rheumatoid synovial fibroblasts after stimulation with TLR3 and TLR4 ligands
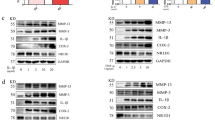
The many different cytokines in the RA joint microenvironment affect the FLS in rheumatoid synovial tissue. Therefore, we investigated the expression of IDO mRNA by rheumatoid synovial fibroblasts after stimulation with various cytokines including IFN-γ, IL-17, TNF-α, IL-12, IL-23 and IL-16. As expected, IDO expression in synovial fibroblasts was increased by stimulation with IFN-γ but not the other cytokines (Figure 1A). Expression of IDO was also assessed by PCR (Figure 1B) and western blot analysis (Figure 1C). IFN-γ-induced IDO mRNA and protein expression were also inhibited in the presence of 1-methyl-tryptophan (1-MT), which is a competitive inhibitor of IDO.
Expression of IDO in rheumatoid synovial fibroblasts after stimulation with various cytokines. (A) Cells were stimulated with IFN-γ (100 U), IL-17 (10 ng/ml), TNF-α (10 ng/ml), IL-12 (10 ng/ml), IL-23 (10 ng/ml) or IL-16 (10 ng/ml) for 24 h. (B and C) Cells were pretreated with 1-MT for 2 h prior to IFN-γ stimulation for 24 h. Total RNA and protein were then isolated from the cells and subjected to real time RT-PCR for IDO mRNA and western blotting for expression of IDO mRNA (B) and protein (C) respectively. Data are means ± SD of replicate samples in one of three experiments. *P < 0.05 and **P < 0.01, treated vs. untreated cells.
As TLRs trigger IDO expression in human gingival fibroblasts (Mahanonda et al., 2007), we evaluated whether IDO expression in synovial fibroblasts could be modulated by TLR3 and TLR4 ligands. Synovial fibroblasts were stimulated with poly (I:C) for 48 h. Poly (I:C) is a ligand for TLR3 and induced IDO protein and mRNA expression (Figures 2A and 2B).
Increased expression of IDO in rheumatoid synovial fibroblasts after stimulation with TLR3 ligand. Cells were stimulated with poly (I:C) (50 and 100 µg/ml) for 24 h. (A) IDO protein (B) IDO or TRIF mRNA levels were analyzed by Western blotting or real time RT-PCR, respectively. Data are presented as means ± SD of replicate samples in one of three experiments. *P < 0.01, treated vs. untreated cells.
In general, there are three possible routes by which TLR signaling is mediated (Akira and Takeda, 2004). These routes involve either the adapter molecule MyD88, the TIR domain-containing adaptor inducing IFN-β (TRIF) or both. TLR3 is known to use only TRIF to mediate poly (I:C)-induced signaling pathways. Thus, we analyzed the expression pattern of TRIF by poly (I:C) in synovial fibroblasts. Expression of TRIF in synovial fibroblasts was increased by stimulation with poly (I:C) in a dose-dependent manner (Figure 2B).
We next investigated the effects of the TLR4 ligand LPS on the expression of IDO in synovial fibroblasts. Similarly to poly (I:C), LPS induced IDO protein and mRNA expression (Figures 3A and 3B). TLR4 utilizes both MyD88 and TRIF to transduce the signal it receives. LPS induced an increased expression of mRNA for both the common adaptor protein MyD88 and the TLR3- and TLR4-specific adaptor protein TRIF in synovial fibroblasts (Figure 3B).
Increased expression of IDO in rheumatoid synovial fibroblasts after stimulation with TLR4 ligand. Cells were stimulated with LPS (0.1 and 1 µg/ml) for 24 h. (A) IDO protein (B) IDO, MyD88 or TRIF mRNA levels were analyzed by Western blotting or real time RT-PCR, respectively. Data are presented as means ± SD of replicate samples in one of three experiments. *P < 0.01 and **P < 0.001, treated vs. untreated cells.
MyD88 blockade causes induction of IDO in synovial fibroblasts
As shown above, IDO was induced in synovial fibroblasts by poly (I:C) and LPS. To further examine the role of the common adaptor protein MyD88, which is downstream from most TLRs, in the induction of IDO, we examined whether transfection of small interfering RNA (siRNA) for MyD88 would affect IDO expression in synovial fibroblasts. The cells were transfected with control or MyD88-specific siRNA for 24 h before stimulation with LPS (Figure 4). Synovial fibroblasts transfected with MyD88 siRNA had a typical cultured fibroblast phenotype (CD14-, CD68- and CD90+, data not shown). MyD88 transcript but not TRIF showed approximately 50% reduction after treatment with MyD88-specific siRNA compared with that in cells treated with control siRNA (Figure 4B).
MyD88 blockade increases IDO induction in synovial fibroblasts after stimulation with LPS. Rheumatoid synovial fibroblasts were transfected with MyD88 siRNA or scrambled siRNA. After 24 h, transfected fibroblasts were stimulated with LPS (1 µg/ml) for 5, 15, 30 min, and 24 h. IκB phosphorylation (A) IDO, MyD88 or TRIF mRNA (B) expressions were examined by western blotting or real time RT-PCR, respectively. Data are presented as means ± SD of replicate samples in one of three experiments. *P < 0.05 and **P < 0.01, MyD88-transfected vs. scrambled-transfected cells.
LPS can activate NF-kB through the MyD88 and NF-kB activity cause the phosphorylation of IkBs. We therefore assessed phosphorylation status of IkB to determine activity of NF-kB in synovial fibroblasts transfected with MyD88 siRNA (Figure 4A). IkB were phosphorylated immediately after LPS exposure and reached a peak in 15 min, and disappeared in 30 min. As compared to control siRNA, silencing of the MyD88 caused the decreased phosphorylation of IkB. Interestingly, siRNA-mediated knockdown of MyD88 resulted in the expression of higher levels of IDO in synovial fibroblasts compared with those treated with control siRNA (Figure 4B).
Increased expression of IDO, TLR3 and TLR4 in synovial tissue from patients with RA
To detect TLR3 and TLR4 expression in synovial inflammation, we examined the level of expression of IDO, TLR3 and TLR4 according to the degree of inflammation in synovial tissue from RA and osteoarthritis (OA) patients (Figure 5A). Although TLR4 expression in severe RA was similar to that in mild RA, IDO and TLR3 expression correlated strongly with the degree of joint inflammation in RA synovial tissue. TLR3 and TLR4 were predominantly located in the lining regions of RA synovial tissue. In contrast with RA tissues, expression of TLR3 and TLR4 was low in synovial tissue from OA patients. IDO was predominantly located in the lining regions of RA synovium compared with synovium from OA patients. We also found that IDO is highly expressed in the basal state in synovial tissue from RA compared with that from OA patients (Figure 5B).
Increased expression of IDO, TLR3 and TLR4 in synovium of patients with rheumatoid arthritis. (A) IDO, TLR3 and TLR4 expression (brown) was detected by immunohistochemistry in synovium of RA patients with severe or mild disease and OA patients. Nuclei were stained by hematoxylin (blue). An isotype control antibody served as negative control (Original magnification × 400). (B) The endogenous expression of IDO in synovial fibroblasts of RA and OA patients was examined by real time RT-PCR. Data are presented as mean ± SD of five independent experiments. *P < 0.05, severe RA vs. mild RA or OA.
Discussion
In this study using rheumatoid synovial fibroblasts, we evaluated the effect of TLR ligands LPS (TLR4 ligand) and poly (I:C) (TLR3 ligand) on the induction of IDO, which mediates immune tolerance. Our results show that poly (I:C) and LPS induce IDO mRNA and protein expression and intriguingly, that selective deletion of MyD88 with siRNA resulted in a remarkable upregulation of IDO expression.
Numerous reports have demonstrated that IDO retards autoimmune disease progression in several models of autoimmune disorders and allergic diseases. Treatment with the IDO inhibitor 1-MT accelerated disease progression in murine models of experimental autoimmune encephalomyelitis, type 1 diabetes and RA (Sakurai et al., 2002; Grohmann et al., 2003; Szanto et al., 2007). IDO can be induced in vitro or in vivo by various agents including cytokines (IFN-γ, TNF-α), CD40L, CTLA4-Ig, influenza virus (Yoshida et al., 1979; Fujigaki et al., 2001; Grohmann et al., 2002; Hwang et al., 2005). Paradoxically, immunostimulatory LPS and unmethylated CpG, which bind to TLR4 and TLR9, respectively, also induced expression of IDO (Munn, 2006). The activation of TLRs-7/8 with immunostimulatory RNAs or R848, a specific ligand for TLR7/8, can induce IDO expression in human monocytes (Furset et al., 2008). It seems likely that IDO expression in sites of inflammation may represent a counterregulatory mechanism to restrain excessive or inappropriate immune activation. In fact, blocking this inflammation-induced IDO expression can substantially worsen local tissue damage, sometimes to the point of lethality (Gurtner et al., 2003).
We and other investigators have shown that TLRs 2, 3, and 4 are expressed in the synovium of patients with longstanding RA (Kim et al., 2007; Ospelt et al., 2008). Endogenous TLR ligands can be found in the inflamed joints of patients with noninfectious forms of arthritis (van der Heijden et al., 2000). In the presence of functional TLRs in synovial tissue, it is conceivable that endogenous TLR ligands might induce IDO in synovial fibroblasts. We reconfirmed that TLR3, TLR4 and IDO were markedly increased in synovial tissue from RA synovium compared with synovial tissue from OA patients. We also showed high endogenous expression of IDO in the synovial fibroblasts of patients with RA. It is possible that the increased IDO expression observed in the synovial environment of patients with RA may be a result of the release of endogenous TLR ligands from the inflamed synovial tissue, which might then further activate synovial fibroblasts via TLRs, rather than be caused by exposure to inflammatory cytokines in the rheumatoid milieu in vivo. However, regardless of the increased IDO activity of the synovium, synovial effector T cells were present with increased frequency in the inflamed synovial environment. A previous study has suggested possible mechanisms contributing to the persistence of autoreactive T cells in local tissues of patients with autoimmune disease (Zhu et al., 2006). In RA, increased tryptophanyl tRNA synthetase expression in synovial T cells allows resistance to IDO-mediated tryptophan deprivation. Increased expression of IDO in the synovial environment of patients with RA is not a sufficient counterregulator to suppress the excessive T cell immune response and consequently permits the persistence of inflammatory synovial T cells.
IDO induction may be regulated by TLR signaling, because the degree of IDO expression was differentially induced by LPS and poly (I:C). However, the exact molecular connection leading to IDO expression following TLR ligand stimulation remains to be addressed. TRIF and MyD88 adaptor molecules mediate the MyD88-independent and MyD88-dependent signaling pathways, respectively, of various TLR family members (Akira and Takeda, 2004). Based on the present study and others (Hissong et al., 1995; Mellor et al., 2005; Furset et al., 2008), it is clear that IDO induction couples MyD88-mediated and TRIF-mediated signaling. TLR3 ligation resulted in approximately threefold higher expression of IDO than the ligation of TLR4. This finding suggests that IDO may be more strongly induced through TRIF-dependent signaling than MyD88-dependent signaling in response to various TLR ligands. TLR3 engages the TRIF-dependent signaling pathway and is a relatively weak inducer of inflammatory cytokines such as TNF-α and IL-1. Instead, TLR3 mediates innate responses against double-stranded RNA viruses by inducing production of large amounts of type I IFNs. Type I IFNs can either promote (Gutterman, 1994) or suppress (Ivashkiv, 2003) immunity and inflammation. IDO expression in synovial fibroblasts may be induced by type I IFN production after TLR3 ligation in a TRIF-dependent signaling mechanism. This is in agreement with a previous study that showed that IDO protein was not detected in TRIF-/- dendritic cells pulsed with a fungus (De Luca et al., 2007). As IDO-expressing dendritic cells are thought to be important in the generation and maintenance of peripheral tolerance via depletion of autoreactive T cells and induction of regulatory T cell responses (Hayashi et al., 2004), our findings reveal an unexpected role for TRIF in the orchestration of immune responses in autoimmune arthritis.
Although as described previously IDO was induced through activation of the TLR7/MyD88 pathway after HIV infection (Manches et al., 2008), the MyD88 pathway was originally thought to mainly involve the early phase of NF-κB and mitogen-activated protein kinase activation, which leads to the production of inflammatory cytokines such as TNF-α and IL-6 (Akira and Takeda, 2004). One interesting observation in the present study was that under conditions of MyD88 deficiency, IDO expression was increased in synovial fibroblasts through the NF-κB pathway in response to LPS, a finding consistent with the observation that yeast increased IDO expression in MyD88-/- mice compared with wild-type mice (De Luca et al., 2007). This finding suggests that excessive MyD88 signaling in proinflammatory environments, as in RA, may promote inflammation by relatively weak expression of IDO for depletion of autoreactive T cells. Therefore, we suggest that MyD88 deficiency can attenuate inflammatory processes and implicate IDO expression as a potential negative regulator of inflammatory diseases via inhibition of pathogenic T cell proliferation.
In summary, our data provide evidence for a link between IDO induction and TLR signaling. We demonstrated that MyD88 deficiency upregulated IDO expression in synovial fibroblasts. Information gained from this result may help us to further develop immunotherapeutic strategies for the treatment of RA. For example, using drugs that selectively block MyD88 may significantly improve the clinical outcomes of RA.
Methods
Reagents
Highly purified TLR ligands including poly (I:C) (TLR3 ligand) and LPS from Escherichia coli (TLR4 ligand) and 1-Methyl-d-tryptophan (1-MT) were purchased from Sigma-Aldrich (St. Louis, MO). IFN-γ, IL-17, TNF-α, IL-12, IL-23 and IL-16 were purchased from R&D Systems (Minneapolis, MN).
Isolation of synovial fibroblasts
Synovial tissue samples were collected from the knee joints of patients who underwent primary total joint replacement surgery. To isolate the fibroblasts, synovial tissues were diced into small pieces and digested overnight with 1 mg/ml type I collagenase (Worthington, Lakewood, NJ). The suspended fibroblasts were cultured in DMEM, 10% heat-inactivated FBS, and 100 units/ml penicillin G and 100 µg/ml streptomycin sulfate (all from Invitrogen, Carlsbad, CA). The fibroblasts were passaged after reaching 90% confluence and used for experiments after 5-6 passages. More than 98% of synovial cells were CD90+ (BD Biosciences, San Diego, CA), and no macrophages (CD11b+ cells) (BD Biosciences) were detected using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and FITC-conjugated monoclonal antibodies.
Quantitative polymerase chain reaction (PCR)
Total cellular RNA from human synovial fibroblasts was extracted with TRIzol reagent (Invitrogen, Burlington, Ontario, Canada) according to the manufacturer's specifications. Two micrograms of total RNA was reverse transcribed by oligo-dT priming using a reverse transcriptase system (Promega, Madison, WI). PCR was performed with primers specific for human IDO, MyD88, TRIF and the housekeeping gene β-actin. The PCR continued for 20 cycles (94℃ for 30 s, 60℃ for 30 s, 72℃ for 30 s, and a final elongation step at 72℃ for 7 min) for β-actin, 32 cycles for IDO, and 30 cycles for MyD88 and TRIF; the annealing temperature was 58℃. The primers were as follows: β-actin upstream, GGACTTCGAGCAAGAGATGG and downstream, TGTGTTGGCGTACAGGTCTTTG; IDO upstream, GCGCTGTTGGAAATAGCTTC and downstream, TTTGGGTCTTCCCAGAACC; MyD88 upstream, TAAGAAGGACCAGCAGAGCC and downstream, CATGTAGTCCAGCAACAGCC; and TRIF upstream, AAGCCATGATGAGCAACCTC and downstream, GTGTCCTGTTCCTTCCTCCAC. Real-time quantification of messenger RNA was performed using a Roche LightCycler 1.5 capillary-based system real-time PCR system (Roche Diagnostics, Almere, Netherlands) in 20 µl of reaction mixture containing 10 µl of SYBR Green PCR Master Mix (Takara, Osaka, Japan), 500 nM of each primer, and 2 µl of sample DNA. The results were expressed as the threshold cycle (Ct) and then calculated as the ratio of the number of molecules of the target gene to the number of molecules of β-actin. Specificity of the amplified products was assessed by melting curve analysis and gel electrophoresis.
Knockdown of MyD88 using siRNA
Human synovial fibroblasts were seeded into 60 mm dishes at a density of 1 × 105 cells per well and grown to 80% confluence. Cells were transfected, using a PolyMAG (Chemicell, Berlin, Germany), with 50 nM of specific MyD88 or negative control siRNA (Dharmacon Inc., Chicago, IL) for 48 h, according to the manufacturer's protocol. Subsequently, the cells were stimulated with LPS for 24 h.
Western blotting
Cells were rinsed in ice-cold phosphate-buffered saline and incubated in lysis buffer composed of 50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 150 mM NaCl, 0.5% Nonidet P-40, 1 mM PMSF and a protease inhibitor cocktail. Extracts were clarified at 14,000 g at 4℃ for 20 min and protein concentration was determined using the Bradford assay. Thirty micrograms of protein were then resolved by one-dimensional 10% SDS-polyacrylamide gel electrophoresis and transferred to Hybond-P polyvinylidene fluoride membranes. Blocking of membranes was carried out using 5% bovine serum albumin in Tris-buffered saline (TBS)-Tween (0.05% Tween 20, 10 mM TBS, pH 7.5) for 2 h at room temperature. Blots were incubated for 16 h at 4℃ with 0.5 µg/ml of rabbit anti-IDO polyclonal antibody (Transgenic Inc., Kobe, Japan) or Rabbit anti-IκB-α antibody (Cell Signaling Technology, Beverly, MA). After washing, membranes were treated with horseradish peroxidase-conjugated anti-mouse secondary antibody for 1 h at room temperature. After washing, signal was developed using an enhanced chemiluminescence system (GE Healthcare, UK) and detected by exposure to X-ray film.
Immunohistochemistry
Immunohistochemical staining was performed on sections of synovium samples obtained from RA and OA patients. The tissues were embedded in optimal cutting temperature compound (Tissue-Tek TT 4583, Sakura Finetech, Torrance, CA), snap frozen in liquid nitrogen, and stored at -80℃. Tissue sections (7 µm) were fixed in 4% paraformaldehyde solution overnight at 4℃. The sections were depleted of endogenous peroxidase activity by adding methanolic H2O2, blocked with normal serum for 30 min, and incubated overnight at 4℃ with antibodies to IDO (Transgenic Inc.), TLR3 or TLR4 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). After the overnight incubation, the samples were incubated with the secondary antibody, biotinylated IgG, for 20 min, with a streptavidin-peroxidase complex (Vector, Peterborough, UK) for 1 h, and then with 3,3'-diaminobenzidine (Dako, Glostrup, Denmark). The sections were counterstained with hematoxylin. Samples were photographed with an Olympus photomicroscope (Tokyo, Japan).
Statistical analysis
Data were obtained from at least three independent experiments. Results are expressed as means ± standard deviation (SD). Student's t-tests were used to determine differences between the two groups.
Abbreviations
- IDO:
-
indoleamine 2,3-dioxygenase
- LPS:
-
lipopolysaccharide
- MyD88:
-
myeloid differentiation primary response gene (88)
- RA:
-
rheumatoid arthritis
- TLR:
-
toll-like receptor
- TRIF:
-
TIR domain-containing adaptor inducing IFN-β
References
Akira S, Takeda K . Toll-like receptor signalling . Nat Rev Immunol 2004 ; 4 : 499 - 511
Beutelspacher SC, Tan PH, McClure MO, Larkin DF, Lechler RI, George AJ . Expression of indoleamine 2,3-dioxygenase (IDO) by endothelial cells: implications for the control of alloresponses . Am J Transplant 2006 ; 6 : 1320 - 1330
Brentano F, Kyburz D, Schorr O, Gay R, Gay S . The role of Toll-like receptor signalling in the pathogenesis of arthritis . Cell Immunol 2005 ; 233 : 90 - 96
Cha HS, Bae EK, Ahn JK, Lee J, Ahn KS, Koh EM . Slug suppression induces apoptosis via Puma transactivation in rheumatoid arthritis fibroblast-like synoviocytes treated with hydrogen peroxide . Exp Mol Med 2010 ; 42 : 428 - 436
De Luca A, Montagnoli C, Zelante T, Bonifazi P, Bozza S, Moretti S, D'Angelo C, Vacca C, Boon L, Bistoni F, Puccetti P, Fallarino F, Romani L . Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc . J Immunol 2007 ; 179 : 5999 - 6008
Fujigaki S, Saito K, Sekikawa K, Tone S, Takikawa O, Fujii H, Wada H, Noma A, Seishima M . Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism . Eur J Immunol 2001 ; 31 : 2313 - 2318
Furset G, Floisand Y, Sioud M . Impaired expression of indoleamine 2,3-dioxygenase in monocyte-derived dendritic cells in response to Toll-like receptor-7/8 ligands . Immunology 2008 ; 123 : 263 - 271
Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P . CTLA-4-Ig regulates tryptophan catabolism in vivo . Nat Immunol 2002 ; 3 : 1097 - 1101
Grohmann U, Fallarino F, Bianchi R, Orabona C, Vacca C, Fioretti MC, Puccetti P . A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice . J Exp Med 2003 ; 198 : 153 - 160
Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF . Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice . Gastroenterology 2003 ; 125 : 1762 - 1773
Gutterman JU . Cytokine therapeutics: lessons from interferon alpha . Proc Natl Acad Sci USA 1994 ; 91 : 1198 - 1205
Hayashi T, Rao SP, Takabayashi K, Van Uden JH, Kornbluth RS, Baird SM, Taylor MW, Carson DA, Catanzaro A, Raz E . Enhancement of innate immunity against Mycobacterium avium infection by immunostimulatory DNA is mediated byindoleamine 2,3-dioxygenase . Infect Immun 2001 ; 69 : 6156 - 6164
Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, Broide DH, Carson DA, Raz E . Inhibition of experimental asthma by indoleamine 2,3-dioxygenase . J Clin Invest 2004 ; 114 : 270 - 279
Hissong BD, Byrne GI, Padilla ML, Carlin JM . Upregulation of interferon-induced indoleamine 2,3-dioxygenase in human macrophage cultures by lipopolysaccharide, muramyl tripeptide, and interleukin-1 . Cell Immunol 1995 ; 160 : 264 - 269
Hwang SL, Chung NP, Chan JK, Lin CL . Indoleamine 2,3-dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines . Cell Res 2005 ; 15 : 167 - 175
Igari T, Tsuchizawa M, Shimamura T . Alteration of tryptophan metabolism in the synovial fluid of patients with rheumatoid arthritis and osteoarthritis . Tohoku J Exp Med 1987 ; 153 : 79 - 86
Ivashkiv LB . Type I interferon modulation of cellular responses to cytokines and infectious pathogens: potential role in SLE pathogenesis . Autoimmunity 2003 ; 36 : 473 - 479
Kim KW, Cho ML, Lee SH, Oh HJ, Kang CM, Ju JH, Min SY, Cho YG, Park SH, Kim HY . Human rheumatoid synovial fibroblasts promote osteoclastogenic activity by activating RANKL via TLR-2 and TLR-4 activation . Immunol Lett 2007 ; 110 : 54 - 64
Maffia P, Brewer JM, Gracie JA, Ianaro A, Leung BP, Mitchell PJ, Smith KM, McInnes IB, Garside P . Inducing experimental arthritis and breaking self-tolerance to joint-specific antigens with trackable, ovalbumin-specific T cells . J Immunol 2004 ; 173 : 151 - 156
Mahanonda R, Sa-Ard-Iam N, Montreekachon P, Pimkhaokham A, Yongvanichit K, Fukuda MM, Pichyangkul S . IL-8 and IDO expression by human gingival fibroblasts via TLRs . J Immunol 2007 ; 178 : 1151 - 1157
Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, Bhardwaj N . HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism . J Clin Invest 2008 ; 118 : 3431 - 3439
Mellor AL, Munn DH . Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation ? Immunol Today 1999 ; 20 : 469 - 473
Mellor AL, Munn DH . Tryptophan catabolism prevents maternal T cells from activating lethal anti-fetal immune responses . J Reprod Immunol 2001 ; 52 : 5 - 13
Mellor AL, Munn DH . IDO expression by dendritic cells: tolerance and tryptophan catabolism . Nat Rev Immunol 2004 ; 4 : 762 - 774
Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH . Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling . J Immunol 2005 ; 175 : 5601 - 5605
Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL . Prevention of allogeneic fetal rejection by tryptophan catabolism . Science 1998 ; 281 : 1191 - 1193
Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL . Inhibition of T cell proliferation by macrophage tryptophan catabolism . J Exp Med 1999 ; 189 : 1363 - 1372
Munn DH, Mellor AL . IDO and tolerance to tumors . Trends Mol Med 2004 ; 10 : 15 - 18
Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL . Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes . J Clin Invest 2004 ; 114 : 280 - 290
Munn DH . Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation . Curr Opin Immunol 2006 ; 18 : 220 - 225
Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, Moqbel R . Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase . J Immunol 2004 ; 173 : 5909 - 5913
Ospelt C, Brentano F, Rengel Y, Stanczyk J, Kolling C, Tak PP, Gay RE, Gay S, Kyburz D . Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis . Arthritis Rheum 2008 ; 58 : 3684 - 3692
Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM . Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis . J Neuroimmunol 2002 ; 129 : 186 - 196
Schroecksnadel K, Winkler C, Duftner C, Wirleitner B, Schirmer M, Fuchs D . Tryptophan degradation increases with stage in patients with rheumatoid arthritis . Clin Rheumatol 2006 ; 25 : 334 - 337
Szanto S, Koreny T, Mikecz K, Glant TT, Szekanecz Z, Varga J . Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice . Arthritis Res Ther 2007 ; 9 : R50 -
Takeda K, Kaisho T, Akira S . Toll-like receptors . Annu Rev Immunol 2003 ; 21 : 335 - 376
Tak PP, Bresnihan B . The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis . Arthritis Rheum 2000 ; 43 : 2619 - 2633
Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ . Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase . Nat Med 2003 ; 9 : 1269 - 1274
van der Heijden IM, Wilbrink B, Tchetverikov I, Schrijver IA, Schouls LM, Hazenberg MP, Breedveld FC, Tak PP . Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides . Arthritis Rheum 2000 ; 43 : 593 - 598
Yoshida R, Urade Y, Tokuda M, Hayaishi O . Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection . Proc Natl Acad Sci USA 1979 ; 76 : 4084 - 4086
Yuan W, Collado-Hidalgo A, Yufit T, Taylor M, Varga J . Modulation of cellular tryptophan metabolism in human fibroblasts by transforming growth factor-beta: selectiveinhibition of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA synthetase gene expression . J Cell Physiol 1998 ; 177 : 174 - 186
Zhu L, Ji F, Wang Y, Zhang Y, Liu Q, Zhang JZ, Matsushima K, Cao Q . Synovial autoreactive T cells in rheumatoid arthritis resist IDO-mediated inhibition . J Immunol 2006 ; 177 : 8226 - 8233
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number 2008-2005645).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Park, MK., Oh, HJ., Heo, YM. et al. Myeloid differentiation primary response protein 88 blockade upregulates indoleamine 2,3-dioxygenase expression in rheumatoid synovial fibroblasts. Exp Mol Med 43, 446–454 (2011). https://doi.org/10.3858/emm.2011.43.8.050
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2011.43.8.050