Abstract
Nonspecific inflammatory response is the major cause for failure of islet grafts at the early phase of intraportal islet transplantation (IPIT). Bilirubin, a natural product of heme catabolism, has displayed anti-oxidative and anti-inflammatory activities. The present study has demonstrated that bilirubin protected islet grafts by inhibiting nonspecific inflammatory response in a syngeneic rat model of IPIT. The inflammation-induced cell injury was mimicked by exposing cultured rat insulinoma INS-1 cells to cytokines (IL-1β, TNF-α and IFN-γ) in in vitro assays. At appropriate lower concentrations, bilirubin significantly attenuated the reduced cell viability and enhanced cell apoptosis induced by cytokines, and protected the insulin secretory function of INS-1 cells. Diabetic inbred male Lewis rats induced by streptozotocin underwent IPIT at different islet equivalents (IEQs) (optimal dose of 1000, and suboptimal doses of 750 or 500), and bilirubin was administered to the recipients every 12 h, starting from one day before transplantation until 5 days after transplantation. Administration of bilirubin improved glucose control and enhanced glucose tolerance in diabetic recipients, and reduced the serum levels of inflammatory mediators including IL-1β, TNF-α, soluble intercellular adhesion molecule 1, monocyte chemoattractant protein-1 and NO, and inhibited the infiltration of Kupffer cells into the islet grafts, and restored insulin-producing ability of transplanted islets.
Similar content being viewed by others
Introduction
Intraportal islet transplantation (IPIT) may be an effective clinical strategy for the cure of type 1 diabetes mellitus (T1DM). Improved islet preparation and immunosuppressive regimens have contributed to increased success rates of IPIT (Shapiro et al., 2003). However, 50-75% of islets fail to engraft at an early phase, representing a major impediment for successful IPIT (Yin et al., 2006). Alloantigen-specific immune-mediated destruction is one of the causes for early islet failure, but nonspecific inflammatory or innate immune response may be more important as shown in a syngeneic mouse model system where approximately 30% of the islets lose their function by day 1 of transplantation (Yin et al., 2006) and as high as 60% by day 3 (Biarnés et al., 2002). In a pig model 50% of islets were destroyed within 20 min after IPIT (Eich et al., 2007). Therefore, inhibiting the nonspecific inflammatory response may protect transplanted islets and increase the success rate of IPIT. There have been several approaches already to improve the survival of transplanted islets by inhibiting the ensuing nonspecific inflammation (Arita et al., 1998; Ozmen et al., 2002; Tran et al., 2002; Moberg et al., 2003; Contreras et al., 2004; Goto et al., 2004; Yang et al., 2005; Jung et al., 2009).
Bilirubin is the final product of heme catabolism (Tenhunen et al., 1968). Accumulating evidence indicates that bilirubin has a beneficial role as a potent anti-oxidant, and clinical data show an inverse correlation between plasma bilirubin levels and various diseases (Vítek et al., 2002, 2006; Wiedemann et al., 2003; Sedlak et al., 2004; Lin et al., 2006). It displays anti-inflammatory activity in animal models of endotoxemia, septicemia and ischemia reperfusion injury (Fondevila et al., 2004; Wang et al., 2004; Keshavan et al., 2005; Lanone et al., 2005; Nakao et al., 2005; Overhaus et al., 2006; Kadl et al., 2007). Some of the proposed mechanisms of bilirubin including its ability to down-regulate expression of adhesion molecules, inhibit infiltration of inflammatory cells and production of nitric oxide (NO), and to reduce production of various inflammatory factors. Thus, its anti-oxidative, anti-inflammatory and cytoprotective properties make bilirubin an attractive candidate for protecting transplanted islets against nonspecific inflammation-induced damage. The present study investigates whether bilirubin could protect islet grafts by inhibiting nonspecific inflammatory response in a syngeneic rat model of IPIT.
Results
Effects of bilirubin on cell viability impaired by cytokines
First, we mimicked nonspecific inflammation-induced cell injury by exposing INS-1 cells to the cytokines, IL-1β, TNF-α and IFN-γ as described previously (Grunnet et al., 2009; Zaitseva et al., 2009). Cell viability was assessed in harvested cells at different time points following cytokine exposure. As shown in Figure 1A, cytokine exposure resulted in reduced cell viability in a time-dependent manner. 48 h exposure to cytokines resulted in 45% inhibition of cell viability.
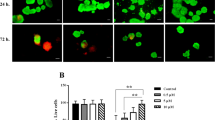
Cell viability and apoptosis. (A) INS-1 cells were cultured in RPMI 1640 media containing murine IL-1β (100 U/ml), TNF-α (500 U/ml) and IFN-γ (100 U/ml) for 16, 24, 32, 40 or 48 h. (B) INS-1 cells were cultured in the same media containing 0, 5, 10, 20, 30 or 50 µM of bilirubin for 1 h, then replaced with cytokine-containing media as above, and cells were further cultured for 48 h. (A, B) Untreated cells served as controls, and the cell viability was measured by CCK-8 assays. *indicates a significant increase of cell viability, and ‡a significant reduction, compared with cytokines only-treated cells. (C) INS-1 cells were cultured in cytokine-containing media as above for 8, 16, or 24 h. (D) INS-1 cells were cultured in media containing 20 or 50 µM BR (bilirubin) for 1 h, then replaced with cytokine-containing media as above, and cells were further cultured for 24 h. (C, D) Untreated cells served as controls, and flow cytometry was performed to measure apoptosis rates. The above experiments were repeated thrice, and results were expressed mean ± SD. *indicates a significant increase of apoptosis rates from control at P < 0.05, and **a highly significant increase from control at P < 0.001. ‡indicates a significant reduction of apoptosis rates from cytokine only-treated cells at P < 0.05. (E) Representative dot plots were from cytometrically analyzed untreated (control) INS-1 cells or INS-1 cells receiving different treatments. The percentage of cell population is shown in each quadrant. (F) Illustrated are representative photographs (× 400 magnification) for untreated (control) INS-1 cells or INS-1 cells subjected to different treatments, stained with Annexin V/PI and examined under laser scanning confocal microscopy to detect apoptotic cells.
Next, we evaluated the protective effects of bilirubin by pre-incubating cells with bilirubin at different concentration for 1 h, and then exposed them to cytokines for 48 h. As shown in Figure 1B, 5 µM of bilirubin did not show a significant protective effect for INS-1 cells compared with the control, while bilirubin at 10 µM significantly inhibited the reduced cell viability induced by cytokines, and at 20 µM showed the strongest inhibition. However, the protective effect of bilirubin at 30 µM was less than that at 20 µM, and at 50 µM, bilirubin even reduced cell viability compared with control samples.
Effects of bilirubin on cell apoptosis induced by cytokines
First, we measured the rate of apoptosis in INS-1 cells exposed to the 3 cytokines for 8, 16 and 24 h. As shown in Figure 1C, exposure to cytokines for 8 h significantly (P < 0.05) increased the apoptotic rate (8.9 ± 2.3%), which increased even more significantly after cytokine exposure for 16 and 24 h (P < 0.001; 18.4 ± 3.5% and 33.4 ± 3.4%, respectively), compared with controls (3.5 ± 1.4%). Next, we pre-incubated INS-1 cells with 20 µM or 50 µM of bilirubin for 1 h, and then exposed them to cytokines for 24 h. As shown in Figure 1D, 20 µM of bilirubin significantly reduced cell apoptosis rate (19.8 ± 2.1%), while 50 µM of bilirubin did not reduce but slightly increased cell apoptosis rate (35.1 ± 2.1%), compared with cells treated with cytokines alone (34.5 ± 3.4%), though the latter difference did not reach significance. Representative dot plots of cytometrically analyzed cells, and photomicrographs of cells stained with Annexin V/PI and examined under laser scanning confocal microscopy are shown in Figures 1E and F, respectively.
Function of INS-1 cells
INS-1 cells were pre-incubated with 20 or 50 µM of bilirubin for 1 h, and then exposed to cytokines for 24 h, and harvested. Their ability to secrete and produce insulin was measured. As shown in Figure 2A, cytokine exposure significantly reduced insulin secretion induced by either low or high glucose. However, pre-treatment with 20 µM but not 50 µM of bilirubin significantly inhibited this reduction, compared with cytokine treatment alone (Figure 2A). This was supported by the alterations of insulin secretion index (Figure 2B), and further by the results of cellular insulin content (Figure 2C). These results suggest that lower concentration (20 µM) but not higher concentration (50 µM) of bilirubin may be the optimal concentration for protecting INS-1 cells against inflammation-mediated cell injury.
Insulin producing activity. The INS-1 cells from Figure 1 (D) were used to measure glucose-stimulated insulin secretion (A), insulin secretion index (B) and intracellular insulin contents (C). The above experiments were repeated thrice, and results were expressed mean ± SD. *indicates a significant decrease from respective control cells at P < 0.05, and ‡indicates a significant increase from respective cells treated by cytokines only at P < 0.05.
Bilirubin improves glucose control in diabetic recipients receiving IPIT
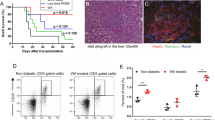
As shown in Figure 3A, diabetes was ameliorated within 5 days in all recipients that were transplanted with 1000 IEQ and treated with either vehicle or bilirubin. However, recipients treated with bilirubin had significantly (all P < 0.05) lower nonfasting glucose levels at indicated time points within 5 days after transplantation, compared with vehicle-treated rats. The difference was more pronounced when recipients received suboptimal islet doses (750 and 500 IEQ) (Figures 3B and C). There were significant differences in nonfasting glucose levels between bilirubin and vehicle-treated recipients receiving 750 or 500 IEQ islets at all the time points 2 days after transplantation. The percentage of recipients with nonfasting glucose < 200 mg/dl is further summarized in Figure 3D. 75% of the recipients receiving bilirubin and IPIT with 750 IEQ had their nonfasting glucose < 200 mg/dl, compared with only 37.5% of the rats treated with vehicle and the same dose of islets (P < 0.05). 12.5% of the recipients receiving bilirubin and 500 IEQ IPIT had their nonfasting glucose < 200 mg/dl, while none of the rats treated with vehicle and the same dose of islets had regained normal glucose levels (P < 0.05).
Bilirubin promotes glucose control and enhances glucose tolerance after IPIT. Streptozotocin-induced diabetic recipients received an optimal islet dose (1000 IEQ/rat) (A) or suboptimal doses (either 750[B] or 500[C] IEQ/rat). Recipients were given bilirubin (1 ml/kg body weight) or vehicle every 12 h, starting one day before and until 5 days after IPIT. Non-fasting glucose levels were assessed at indicated time points. (D) Percentage of recipients that achieved normal glucose level (< 200 mg/dl) in each group in (A, B and C). (E) 30 days after transplantation, the recipients were subjected to glucose tolerance test by fasting overnight and receiving i.p. injection of dextrose (2 g/kg body weight). Blood glucose levels were measured just before injection and 30, 60, 90 and 120 min after the injection. n, the number of rats assessed. *indicates a significant decrease from the respective vehicle-treated groups at respective time points at P < 0.05.
To further asses the function of transplanted islets, we performed glucose tolerance tests in recipients 30 days after transplantation. As shown in Figure 3E, there was no significant difference in blood glucose levels at all the indicated time points between rats receiving 1000 IEQ IPIT treated with bilirubin or vehicle. However, glucose levels in rats receiving 750 IEQ or 500 IEQ IPIT and treated with bilirubin were significantly lower (all P < 0.05) than those treated with vehicle, 60, 90 and 120 min after glucose challenge.
Administration of bilirubin inhibits inflammation induced by IPIT
It has been reported that IPIT induces nonspecific inflammation, resulting in higher levels of inflammatory factors. Accordingly, we have shown that IPIT led to higher serum levels of IL-1β, TNF-α, sICAM-1, MCP-1 and NO at most time points. However, administration of bilirubin reduced these increases (Table 1).
We have also demonstrated that administration of bilirubin inhibited infiltration of Kupffer cells in transplanted islets, and protected their insulin-secreting ability. As shown in Figure 4A, IPIT induced infiltration of more Kupffer cells stained positive with an anti-CD68 Ab 24 h after transplantation, but administration of bilirubin inhibited this accumulation (Figure 4B). The density of CD68-positive cells in islets was significantly lower in bilirubin-treated rats compared with vehicle-treated animals (2.7 ± 1.8% versus 15.5 ± 8.4%; P < 0.05).
Infiltration of Kupffer cells and insulin production by islets in situ. Representative illustrations were taken from livers of vehicle (A, C) or bilirubin (B, D)-treated diabetic rats receiving intraportal injection of 1000 IEQ islets/rat and harvested 24 h (A, B) or 10 days (C, D) later. Each group had 10 rats, five of which were randomly sacrificed 24 h or 10 days after transplantation. The liver sections were immunostained with an anti-CD68 Ab to detect Kupffer cells (A, B), or an anti-insulin Ab to detect insulin (C, D). Brown color indicates positive cells.
Furthermore, we immunostained liver sections taken from recipients 10 days after transplantation with an anti-insulin Ab, and found the islet grafts from recipients treated with bilirubin (Figure 4D) had stronger insulin-staining than those from vehicle-treated recipients (Figure 4C).
Discussion
The present study, has for the first time, demonstrated that bilirubin at an appropriate concentration or dose attenuated the cellular injury in rat insulinoma INS-1 cells induced by cytokines in vitro, and in rats protected intraportally transplanted islets against non-specific inflammation, improved glucose control and promoted glucose tolerance in synegeic diabetic recipients.
The instant blood-mediated nonspecific inflammatory response (IBMIR) markedly affects the outcome of IPIT, and represents a major obstacle for successful IPIT (Biarnés et al., 2002). IBMIR involves activation of coagulation and complement systems, which in turn leads to local ischemia and injury of endothelium and upregulation of ICAM-1 and MCP-1. ICAM-1, a cell-surface molecule involved in immune processes, regulates adhesion of immune cells to islet endothelium (Cantaluppi et al., 2006). MCP-1 promotes infiltration of inflammatory cells, resulting in the production of inflammatory cytokines (Schröppel et al., 2005). Accordingly, this study has revealed the increased serum levels of IL-1β, TNF-α, sICAM-1, MCP-1 and NO, and increased macrophage infiltration into transplanted islets after IPIT.
Although the mechanisms for the protective effects of bilirubin on islet grafts are not entirely clear, it is known to display anti-oxidative, anti-inflammatory and cytoprotective activities. It has been reported that bilirubin has a strong anti-oxidative activity (Liu et al., 2003; Sedlak et al., 2004), and inhibits NOS expression and NO production in response to endotoxin in rats (Wang et al., 2004). The iNOS-NO system plays an important role in acute and severe hypoxic injury in pancreatic β cells (Ko et al., 2008). Therefore, the protective effects of bilirubin may partially depend on its anti-oxidative ability since it inhibits the production of NO elevated by IPIT as demonstrated in the present study. However, IPIT does not induce classical ischemia reperfusion as the islets only induce local ischemia without the process of reperfusion. Our results also showed that bilirubin inhibited the production of IL-1β, TNF-α, sICAM-1 and MCP-1. Notably, its precursor, biliverdin, exhibited anti-inflammatory properties by inhibiting the activity of NF-κB in a rat model of endotoxin-induced acute lung injury (Sarady-Andrews et al., 2005). IL-1β and TNF-α have been shown to participate in pancreatic islet β-cell death and functional impairment of β-cells causing early loss of islet mass in transplanted islets (Amoli et al., 2006) and IL-1β and TNF-α blockade promoted the survival of islet grafts (Satoh et al., 2007).
Resident macrophages (Kupffer cells) in the liver play a critical role in mediating nonspecific inflammation after IPIT. In an animal model of IPIT, transient inhibition of Kupffer cells has been found to reduce levels of proinflammatory mediators (TNF-α, IL-β, NO) 3-6 h after transplantation, resulting in improved islet engraftment (Bottino et al., 1998). Here, we have shown that administration of bilirubin inhibited the accumulation of Kuppfer cells in the transplanted islets.
Recent evidence indicates that IBMIR may be triggered, in large part, by islets in direct contact with blood (Moberg et al., 2002; Johansson et al., 2005; Moberg, 2005). IPIT activates factor X to convert prothrombin to thrombin, a key mediator of thrombotic and inflammatory events. Local thrombin induces platelet activation and adhesion, further amplifying coagulation cascades, and ultimately entrapping islets within fibrin clots. Furthermore, thrombin acts as a chemoattractant and can trigger expression of endothelial cell adhesion molecules, promoting migration of neutrophils, monocytes, and Kupffer cells to the portal bed. Additionally, islets release a number of inflammatory mediators to exacerbate thrombotic and inflammatory responses through activation of endothelial cells and attraction and activation of leukocytes (Wilson and Chaikof, 2008). Bilirubin has been found to be as effective as hemin in delaying ferric chloride-induced thrombus formation (Lindenblatt et al., 2004). Therefore, bilirubin's anti-coagulative activity may also contribute to its protective effects on transplanted islets.
In addition, bilirubin has been shown to induce immune tolerance to islet transplants (Wang et al., 2006; Lee et al., 2007; Rocuts et al., 2010). Recent clinical data have shown that high serum bilirubin is associated with a reduced risk of diabetes mellitus and diabetic nephropathy, suggesting bilirubin may have an insulin-sensitizing role (Han et al., 2010). These studies have added further support for the potential use of bilirubin co-therapy for improving the survival of islets transplanted in patients. However, the toxicity of bilirubin should also be taken into consideration, as its neuronal toxicity has long been recognized in infants (Wennberg, 2008). We have used a low dose of bilirubin (8.5 µmol/kg body weight every 12 h) over only 6 days in the present animal experiments. Administration of bilirubin at this dose could only lead to transient increase of bilirubin in the sera for several hours in the animals (Wang et al., 2006). In our in vitro experiments, 20 µM of bilirubin had shown the strongest protective activity, but 50 µM showed toxicity towards INS-1 cells. These results imply bilirubin has a relatively narrow therapeutic window in respect to dosage. Therefore, the preclinical hurdles must be overcome before bilirubin reaches the clinics for use in human islet transplantation.
Methods
Cell culture conditions
The rat insulinoma INS-1 cell line was purchased from the Center of Typical Culture Collection in China (Wuhan, China). The cells were routinely cultured in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 2 mM L-glutamine, 10 mM HEPES, 100 U/ml penicillin, 100 mg/L streptomycin, 1 mM sodium pyruvate, 10% heat-inactivated newborn calf serum, and 50 µM β-mercaptoethanol (Sigma), in a humidified incubator at 95% air and 5% CO2 at 37℃. INS-1 cells (4 × 103/well) were pre-incubated in RPMI1640 medium containing bilirubin (Sigma, Chemical Co, St. Louis, MO) at different concentrations in 96-well plates for 1 h, and then the medium was replaced with RPMI1640 medium containing the murine cytokines, interleukin (IL)-1β (100 U/ml), tumor necrosis factor (TNF)-α (500 U/ml) and interferon (IFN)-γ (100 U/ml) (Peprotech Inc. Rocky Hill, New Jersey), to mimick non-specific inflammation. The cells were further cultured and harvested for analysis.
Cell viability and apoptosis
Cell viability was assayed by a colorimetric procedure with a cell counting kit-8 (CCK-8, Dojindo Laboratories, Japan). 10 µl of CCK-8 was added to each well, and the cells were incubated for 2 h, and the absorbance was determined at 450 nm. Cell viability was calculated as OD of experimental groups/OD of control group × 100%.
Cell apoptosis was examined with the Annexin V-FITC/PI Apoptosis Assay Kit (BD Biosciences, San Jose, CA) according to the manufacturer's protocol. Cells were then analyzed in a Beckman Coulter Epics Altra II cytometer (Beckman Coulter, CA) to measure the apoptosis rate (%), and viewed by laser scanning confocal microscopy (LSM-510, Carl Zeiss Jena GmbH, Jena, Germany).
Cell function tests
Cells were incubated in glucose-free Krebs-Ringer bicarbonate (KRB, Sigma) for 1 h, followed by further incubation with low glucose KRB (5.56 mM of glucose) or high glucose KRB (25 mM of glucose) for 1 h, respectively. Insulin concentration in the media was measured using an insulin RIA kit (Linco Research, St. Charles, MO) and standardized based on total intracellular protein content measured with a BCA protein assay kit (Beyotime Inst. Biotech, Peking, PR China). Insulin secretion index was calculated as insulin concentration in high glucose KRB/in low glucose KRB.
The intracellular insulin content was measured by incubating the cells in 1% hydrochloric acid alcohol overnight at 4℃. After centrifugation, the supernatants were collected, and the concentration of insulin in the supernatants was measured as above, and standardized based on total intracellular protein content.
Animals and induction of diabetes
Adult inbred male Lewis rats (200-250 g) were supplied by Vital River Lab Animal Technology Co, Ltd (Beijing, China). All surgical procedures and care administered to the animals were approved by the institutional ethics committee. After fasting for 12 h, the rats were i.p. injected with 1% streptozotocin (STZ, Sigma) at a dose of 70 mg/kg body weight. Four days later, rats with non-fasting glucose levels higher than 350 mg/dl in two consecutive measurements were used as recipients of islet grafts.
Islet isolation and transplantation
Pancreas was perfused with 10 ml collagenase V (Sigma) at 1 mg/ml via the common bile duct and digested for 13 min in a water bath at 37℃. Islets were separated using density gradient centrifugation. Islet purity was assessed by dithizone (Sigma) staining after isolation. The total number of islets in each diameter class was counted using an optical graticule. The number was then converted to the standard number of islet equivalents (IEQ, diameter standardizing to 150 µm). Islet viability was assessed using fluorescence staining with acridine orange and propidium iodide (Sigma). Islet isolations with > 90% viability and > 90% purity were used. After isolation, islets were washed three times and suspended in HBSS for IPIT.
Animal experiment design
Bilirubin (Sigma) was dissolved to make injectable solution at a concentration of 8.5 mM as described previously (Wang et al., 2006). Diabetic recipients were randomly divided into three groups: Sham, IPIT and IPIT + BR. Rats in the IPIT group received intraportal injection of islets at 1000 (optimal dose), or 750 or 500 IEQ/rat (suboptimal doses), and i.p. injection of vehicle (1 ml/kg bodyweight). The rats in the IPIT + BR group received intraportal injection of islets at 1000, or 750 or 500 IEQ/rat, and i.p. injection of bilirubin (8.5 mM) at a dose of 1 ml/kg body weight (equal to 8.5 µmol/kg body weight). Bilirubin or vehicle was administered every 12 h, starting from one day before transplantation until 5 days after transplantation. Rats in the sham group underwent laparotomy without intraportal or i.p. injections.
Evaluation of islet function after transplantation
Blood glucose levels of the recipients were measured with a glucometer (Roche, Shanghai, China). Animals with non-fasting blood glucose levels less than 200 mg/dl were considered normoglycemic.
Glucose tolerance test was performed 30 days after IPIT. Recipients were fasted overnight and dextrose was i.p. injected at a dose of 2 g/kg body weight. Blood glucose levels were measured before and 30, 60, 90 and 120 min after the injection.
Inflammatory cytokine analysis
Blood samples were obtained from tail vein 3, 6, 12 and 24 h after IPIT. Samples were centrifuged to harvest sera, which were stored at -80℃ until use. The levels of IL-1β, TNF-α, monocyte chemoattractant protein-1 (MCP-1) and soluble intercellular cell adhesion molecule 1 (sICAM-1) were measured with enzyme-linked immunosorbent assay kits (BioSource International, Camarillo, CA) according to the manufacturer's manual. The levels of NO were measured by determining nitrate reductase activity with an NO detection kit (Nanjing Science and Technology Co, Ltd, Nanjing, China) according to the manufacturer's manual.
Histological examination
Liver specimens were fixed in 10% buffered formalin and embedded in paraffin. Five-micron-thick sections were prepared, stained with hematoxylin and eosin, and examined microcopically.
Immunohistochemistry
Liver sections containing transplanted islets were prepared, blocked with 3% BSA, and incubated overnight with rabbit anti-mouse CD68 and anti-insulin primary Abs (Biosynthesis Biotechnology Co, Ltd, Beijing, China), respectively. They were subsequently incubated with the corresponding secondary Abs using the Ultra Sensitive TMS-P kit (Zhongshan Co., Beijing, China), and immunoreactivity developed with Sigma FAST DAB (3,3'-diaminobenzidine tetrahydrochloride) and CoCl2 enhancer tablets (Sigma-Aldrich, Shanghai, China). Sections were counterstained with hematoxylin, mounted, and examined by microscopy. The CD68-positive cells were counted from randomly selected 15 islets per animal, and each group had five animals, thus totally 75 islets were examined. The density of CD68-positive cells (%) was calculated based on the formula: number of CD68 + cells/total cell numbers per islet section area × 100.
Statistical analysis
Results were expressed as mean ± standard deviation (SD), and an analysis of variance (ANOVA) and Dunnett t test were used to evaluate statistical significance. P values < 0.05 were considered statistically significant.
Abbreviations
- BR:
-
bilirubin
- IBMIR:
-
blood-mediated nonspecific inflammatory response
- IEQ:
-
islet equivalents
- IPIT:
-
intraportal islet transplantation
- KRB:
-
Krebs-Ringer bicarbonate
- MCP-1:
-
monocyte chemoattractant protein-1
- sICAM-1:
-
soluble intercellular cell adhesion molecule 1
- STZ:
-
streptozotocin
- T1DM:
-
type 1 diabetes mellitus
References
Amoli MM, Larijani B . Would blockage of cytokines improve the outcome of pancreatic islet transplantation ? Med Hypotheses 2006 ; 66 : 816 - 819
Arita S, Une S, Ohtsuka S, Atiya A, Kasraie A, Shevlin L, Mullen Y . Prevention of primary islet isograft nonfunction in mice with pravastatin . Transplantation 1998 ; 65 : 1429 - 1433
Biarnés M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E . Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia . Diabetes 2002 ; 51 : 66 - 72
Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, Inverardi L . Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation . Diabetes 1998 ; 47 : 316 - 323
Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Ninniri MS, Galimi F, Romagnoli R, Franchello A, Salizzoni M, Perin PC, Ricordi C, Segoloni GP, Camussi G . Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: relevance for islet transplantation . Am J Transplant 2006 ; 6 : 2601 - 2611
Contreras JL, Eckstein C, Smyth CA, Bilbao G, Vilatoba M, Ringland SE, Young C, Thompson JA, Fernàndez JA, Griffin JH, Eckhoff DE . Activated protein C preserves functional islet mass after intraportal transplantation: a novel link between endothelial cell activation, thrombosis, inflammation, and islet cell death . Diabetes 2004 ; 53 : 2804 - 2814
Eich T, Eriksson O, Sundin A, Estrada S, Brandhorst D, Brandhorst H, Langstrom B, Nilsson B, Korsgren O, Lundgren T . Positron emission tomography: a real-time tool to quantify early islet engraftment in a preclinical large animal model . Transplantation 2007 ; 84 : 893 - 898
Fondevila C, Shen XD, Tsuchiyashi S, Yamashita K, Csizmadia E, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Bach FH . Biliverdin therapy protects rat livers from ischemia and reperfusion injury . Hepatology 2004 ; 40 : 1333 - 1341
Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B . Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets . Transplantation 2004 ; 77 : 741 - 747
Grunnet LG, Aikin R, Tonnesen MF, Paraskevas S, Blaabjerg L, Størling J, Rosenberg L, Billestrup N, Maysinger D, Mandrup-Poulsen T . Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells . Diabetes 2009 ; 58 : 1807 - 1815
Han SS, Na KY, Chae DW, Kim YS, Kim S, Chin HJ . High serum bilirubin is associated with the reduced risk of diabetes mellitus and diabetic nephropathy . Tohoku J Exp Med 2010 ; 221 : 133 - 140
Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Källen R, Salmela K, Tibell A, Tufveson G, Ekdahl KN, Elgue G, Korsgren O, Nilsson B . Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation . Diabetes 2005 ; 54 : 1755 - 1762
Jung DY, Park JB, Joo SY, Joh JW, Kwon CH, Kwon GY, Kim SJ . Effect of nicotinamide on early graft failure following intraportal islet transplantation . Exp Mol Med 2009 ; 41 : 782 - 792
Kadl A, Pontiller J, Exner M, Leitinger N . Single bolus injection of bilirubin improves the clinical outcome in a mouse model of endotoxemia . Shock 2007 ; 28 : 582 - 588
Keshavan P, Deem TL, Schwemberger SJ, Babcock GF, Cook-Mills JM, Zucker SD . Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration . J Immunol 2005 ; 174 : 3709 - 3718
Ko SH, Ryu GR, Kim S, Ahn YB, Yoon KH, Kaneto H, Ha H, Kim YS, Song KH . Inducible nitric oxide synthase-nitric oxide plays an important role in acute and severe hypoxic injury to pancreatic beta cells . Transplantation 2008 ; 85 : 323 - 330
Lanone S, Bloc S, Foresti R, Almolki A, Taillé C, Callebert J, Conti M, Goven D, Aubier M, Dureuil B, El-Benna J, Motterlini R, Boczkowski J . Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats . FASEB J 2005 ; 19 : 1890 - 1892
Lee SS, Gao W, Mazzola S, Thomas MN, Csizmadia E, Otterbein LE, Bach FH, Wang H . Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells . FASEB J 2007 ; 21 : 3450 - 3457
Lin JP, O'Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, Yang S, Kronenberg F . Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study . Circulation 2006 ; 114 : 1476 - 1481
Lindenblatt N, Bordel R, Schareck W, Menger MD, Vollmar B . Vascular heme oxygenase-1 induction suppresses microvascular thrombus formation in vivo . Arterioscler Thromb Vasc Biol 2004 ; 24 : 601 - 606
Liu Y, Zhu B, Wang X, Luo L, Li P, Paty DW, Cynader MS . Bilirubin as a potent antioxidant suppresses experimental autoimmune encephalomyelitis: implications for the role of oxidative stress in the development of multiple sclerosis . J Neuroimmunol 2003 ; 139 : 27 - 35
Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Källen R, Østraat Ø, Salmela K, Tibell A, Tufveson G, Elgue G, Nilsson Ekdahl K, Korsgren O, Nilsson B . Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation . Lancet 2002 ; 360 : 2039 - 2045
Moberg L, Olsson A, Berne C, Felldin M, Foss A, Källen R, Salmela K, Tibell A, Tufveson G, Nilsson B, Korsgren O . Nicotinamide inhibits tissue factor expression in isolated human pancreatic islets: implications for clinical islet transplantation . Transplantation 2003 ; 76 : 1285 - 1288
Moberg L . The role of the innate immunity in islet transplantation . Ups J Med Sci 2005 ; 110 : 17 - 55
Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE, Murase N . Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both . Am J Transplant 2005 ; 5 : 282 - 291
Overhaus M, Moore BA, Barbato JE, Behrendt FF, Doering JG, Bauer AJ . Biliverdin protects against polymicrobial sepsis by modulating inflammatory mediators . Am J Physiol Gastrointest Liver Physiol 2006 ; 290 : G695 - G703
Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B . Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation . Diabetes 2002 ; 51 : 1779 - 1784
Rocuts F, Zhang X, Yan J, Yue Y, Thomas M, Bach FH, Czismadia E, Wang H . Bilirubin promotes de novo generation of T regulatory cells . Cell Transplant 2010 ; 19 : 443 - 451
Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, Choi AM, Otterbein LE . Biliverdin administration protects against endotoxin-induced acute lung injury in rats . Am J Physiol Lung Cell Mol Physiol 2005 ; 289 : L1131 - L1137
Satoh M, Yasunami Y, Matsuoka N, Nakano M, Itoh T, Nitta T, Anzai K, Ono J, Taniguchi M, Ikeda S . Successful islet transplantation to two recipients from a single donor by targeting proinflammatory cytokines in mice . Transplantation 2007 ; 83 : 1085 - 1092
Schröppel B, Zhang N, Chen P, Chen D, Bromberg JS, Murphy B . Role of donor-derived monocyte chemoattractant protein-1 in murine islet transplantation . J Am Soc Nephrol 2005 ; 16 : 444 - 451
Sedlak TW, Snyder SH . Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle . Pediatrics 2004 ; 113 : 1776 - 1782
Shapiro AM, Ricordi C, Hering B . Edmonton's islet success has indeed been replicated elsewhere . Lancet 2003 ; 362 : 1242 -
Tenhunen R, Marver HS, Schmid R . The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase . Proc Natl Acad Sci USA 1968 ; 61 : 748 - 755
Tran PO, Gleason CE, Robertson RP . Inhibition of interleukin- 1beta-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet beta-cell function . Diabetes 2002 ; 51 : 1772 - 1778
Vítek L, Jirsa M, Brodanová M, Kalab M, Marecek Z, Danzig V, Novotný L, Kotal P . Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels . Atherosclerosis 2002 ; 160 : 449 - 456
Vítek L, Novotný L, Sperl M, Holaj R, Spácil J . The inverse association of elevated serum bilirubin levels with subclinical carotid atherosclerosis . Cerebrovasc Dis 2006 ; 21 : 408 - 414
Wang H, Lee SS, Dell'Agnello C, Tchipashvili V, d'Avila JC, Czismadia E, Chin BY, Bach FH . Bilirubin can induce tolerance to islet allografts . Endocrinology 2006 ; 147 : 762 - 768
Wang WW, Smith DL, Zucker SD . Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats . Hepatology 2004 ; 40 : 424 - 433
Wennberg R . Unbound bilirubin: a better predictor of kernicterus ? Clin Chem 2008 ; 54 : 207 - 208
Wiedemann M, Kontush A, Finckh B, Hellwege HH, Kohlschütter A . Neonatal blood plasma is less susceptible to oxidation than adult plasma owing to its higher content of bilirubin and lower content of oxidizable Fatty acids . Pediatr Res 2003 ; 53 : 843 - 849
Wilson JT, Chaikof EL . Thrombosis and inflammation in intraportal islet transplantation: a review of pathophysiology and emerging therapeutics . J Diabetes Sci Technol 2008 ; 2 : 746 - 759
Yang Z, Chen M, Ellett JD, Carter JD, Brayman KL, Nadler JL . Inflammatory blockade improves human pancreatic islet function and viability . Am J Transplant 2005 ; 5 : 475 - 483
Yin D, Ding JW, Shen J, Ma L, Hara M, Chong AS . Liver ischemia contributes to early islet failure following intraportal transplantation: benefits of liver ischemic-preconditioning . Am J Transplant 2006 ; 6 : 60 - 68
Zaitseva II, Hultcrantz M, Sharoyko V, Flodström-Tullberg M, Zaitsev SV, Berggren PO . Suppressor of cytokine signaling-1 inhibits caspase activation and protects from cytokine- induced beta cell death . Cell Mol Life Sci 2009 ; 66 : 3787 - 3795
Acknowledgements
This work was supported in part by grants from the Ministry of Science and Technology, China (2007BAI07A05), and the National Natural Scientific Foundation of China (30972938, 30872987 and 30973474). H. Zhu and J. Wang contributed equally to this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhu, H., Wang, J., Jiang, H. et al. Bilirubin protects grafts against nonspecific inflammation-induced injury in syngeneic intraportal islet transplantation. Exp Mol Med 42, 739–748 (2010). https://doi.org/10.3858/emm.2010.42.11.075
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2010.42.11.075