Abstract
Mesenchymal stem cells (MSCs) secrete bioactive factors that exert diverse responses in vivo. In the present study, we explored mechanism how MSCs may lead to higher functional recovery in the animal stroke model. Bone marrow-derived MSCs were transplanted into the brain parenchyma 3 days after induction of stroke by occluding middle cerebral artery for 2 h. Stoke induced proliferation of resident neural stem cells in subventricular zone. However, most of new born cells underwent cell death and had a limited impact on functional recovery after stroke. Transplantation of MSCs enhanced proliferation of endogenous neural stem cells while suppressing the cell death of newly generated cells. Thereby, newborn cells migrated toward ischemic territory and differentiated in ischemic boundaries into doublecortin+ neuroblasts at higher rates in animals with MSCs compared to control group. The present study indicates that therapeutic effects of MSCs are at least partly ascribed to dual functions of MSCs by enhancing endogenous neurogenesis and protecting newborn cells from deleterious environment. The results reinforce the prospects of clinical application using MSCs in the treatment of neurological disorders.
Similar content being viewed by others
Introduction
Stem cell therapies based on its multipotent capacities are emerging as candidates for treatment of various diseases including neuronal or non-neuronal dysfunctions. Neural stem cells (NSCs) or embryonic stem cells (ESCs)-derived neural stem cells have been used for the treatment of neurological dysfunctions such as spinal cord injury (Teng et al., 2002), amyotrophic lateral sclerosis (Silani et al., 2004), and stroke (Park et al., 2002; Kelly et al., 2004; Hayashi et al., 2006). While those approaches have demonstrated that the survival and differentiation of grafted cells into neural cells correlate with behavioral improvement, these cells have limitation for clinical application because of possible immune rejection, insufficient cell supply, and ethical concerns. Moreover, the risks associated with ESCs such as formation of teratoma, are yet to be conquered. Mesenchymal stem cells (MSCs) are bone-marrow derived stem cells that can differentiate into mesodermal tissues, such as fat, bone, cartilage, and muscle (Pittenger et al., 1999). Recently, transdifferentiation of MSCs into neural cells using a variety of growth conditions were reported, although the distinct real transdifferentiation was not achieved (Woodbury et al.,2000; Kohyama et al., 2001; Kwak et al., 2006).
For the regenerative or protective therapy in the stroke treatment, MSCs are appeared as candidate that supply large amounts of angiogenic, antiapoptotic, and mitogenic factors as well as migration toward damaged tissue and neuronal differentiation of themselves, although the specific mechanism remains controversial and needs to be explored (Wang et al., 2002; Chen et al., 2003a; Lee et al., 2007). Therefore, theses cells are considered as candidates for their possible use as autograftable cellular vehicles for both cell and gene therapy. Very recently, MSCs are used in clinical treatment and shown to be effective in the various pathological cares, although an absence of the evidence for distinct therapeutic mechanisms (Fouillard et al., 2002; Wollert et al., 2004; Lee et al., 2007).
Proliferation of resident NSCs in the subventricular zone (SVZ) and subgranular zone (SGZ) involves precise coordination of cell-cycle exit, cell proliferation, cell migration, and initiation of neuronal differentiation (Doetsch et al., 1997; Lee et al., 2006). Although the innate function of persistent neurogenesis from SVZ is poorly understood, recent studies have demonstrated that stroke enhances the SVZ cell proliferation, and that these cells differentiate into mature striatal neurons and replace damaged neurons (Thored et al., 2006). However, since the number of cells generated from neurogenic niche is too low to have a significant impact on functional recovery after stroke, many experimental trials have been undertaken to enhance endogenous neurogenesis after stroke using various agents, such as, EGF, VEGF erythropoietin (EPO), and statins (Chen et al., 2003b; Sun et al., 2003; Teramoto et al., 2003; Wang et al., 2004). Recently, it is reported that transplantation of MSCs enhance functional recovery in animal stroke model as well as endogenous neurogenesis (Chen et al., 2003a; Munoz et al., 2005). However, it has been poorly explored whether the migration, survival, and differentiation of newborn cells generated from NSCs in the SVZ are influenced by transplanted MSCs.
In the present study, we show the intracranial transplantation of MSCs have therapeutic effects in the animal stroke model. We first verify that transplantation of MSCs improved the functional recovery and then prove that not only proliferation of resident NSCs in the SVZ but also the survival of new born neuroblasts are increased by MSCs.
Materials and Methods
Cell culture
All experimental protocols using MSCs were approved by the Institutional Review Board of Ajou University Medical Center (Suwon, Korea). Human MSCs were isolated from bone marrow aspirates as described previously (Kim et al., 2005). MSCs were cultured in DMEM, containing 10% FBS, 100 U penicillin, 100 mg/ml streptomycin (Invitrogen, Grand Island, NY) and 10 ng/ml of basic fibroblast growth factor (Dong-A Pharmaceutical Co., Youngin, Korea). To determine the potential for MSCs to differentiate into mesodermal lineage such as chondrocytes, osteocytes and adipocytes, differentiation was induced according to the procedures described by Pittenger et al. (1999).
Induction of stroke and transplantation of MSCs
All animal protocols were approved by the Institutional Animal Care and Use Committee of Ajou University Medical School. Anesthesia of adult male Sprague-Dawley rats weighing 250-270 g was induced with 5% isoflurane in 70% of N2O and 30% of O2 using a induction chamber and maintained at 3% isoflurane using a face mask. Rectal temperature was maintained at 37℃ throughout the surgical procedure, using an electronic temperature controller linked to a heating pad (FHC, Bowdoinham, ME). Transient middle cerebral artery occlusion (MCAo) was induced as described by Longa et al. (1989) with a slight modification. Briefly, the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were exposed through a ventral midline incision. A 4-0 monofilament nylon suture with a rounded tip was introduced into the CCA lumen and gently advanced into the ICA until it blocked the bifurcating origin of the MCA. Two hours after occlusion, animals were reanesthetized and reperfused by withdrawing the suture until the tip cleared the lumen of CCA. To obtain the rats having a uniformed ischemic injuries, double screen-out method was employed. First, the rats having negligible or moderate ischemic symptoms in any type of behavior tests on day 1 were discarded. Second, the rats having infarct in a small or in a region such as only striatum or cortex were discarded after MRI analysis on day 2. Animals showing similar behavior symptoms and comparable infarct volumes as seen on MRI were selected and randomly grouped to receive PBS or MSCs.
Three days after MCAo, the animals were anesthetized as described above and received MSCs or PBS. After the skull was opened in stereotactic apparatus (Kopf Instruments, Tujunga, CA), 5.0 × 105 of MSCs in 7 µl of PBS were injected into the striatum (AP, 0.5; ML, 2.5; DV, 5.0) and cortex (AP, -0.5; ML, 2.0; DV, 2.5) in the penumbra ipsilateral to the injury for 15 min. Equal volume of PBS was used as a control.
Behavior tests
All animals were pre-trained for 1 week before induction of MCAo. The behavior tests were composed of the tests to measure motor and sensory behavior including rotarod test and adhesive removal test and performed on 1, 7, 14, and 28 days after MCAo. Only the animals capable of remaining on the rotarod cylinder for more than 300 s and removing adhesive dots within 10 s were used for experiments. In the rotarod motor test, the rotarod cylinder (Ugobasile, Comerio, VA, Italy) was accelerated from 4 rpm to 40 rpm within 5 min, and the amount of time that each animal remained on the rotarod was measured with a cut off time of 300 s. The data are presented as percentage of the mean duration from 3 trials with respect to the performance before surgery. For adhesive removal tests, square dots of adhesive-backed paper (100 mm2) were used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb. The time taken for each animal to remove each stimulus was recorded, and animals were given 3 trials with a cut off time of 300 s. The data are presented as the mean time to remove the dots.
Magnetic resonance imaging (MRI)
MRI scanning was performed using a 3.0 Tesla whole body MRI scanner (Magnus 3.0, Medinus Inc., Yongin, Korea) equipped with a gradient system capable of 35 mT/m on 2, 14, 28 days after MCAo. A fast-spin echo imaging sequence was used to acquire T2-weighted anatomical images of the rat brain in vivo, using the following parameters: repetition time = 4,000 ms, effective echo time = 96 ms, field of view = 55 × 55 mm2, image matrix = 256 × 256, slice thickness = 1.5 mm, flip angle = 90°, number of excitations = 2, pixel size = 0.21 × 0.21 mm2. A total of 15 slices were scanned to cover the whole rat brain. For radio frequency irradiation and signal detection, decoupled home-made coils were used. A 300 mm diameter quadrature 16-rung birdcage coil arrangement was used for RF excitation, and a 40 mm diameter saddle coil was used for signal detection. For each slice, the ischemic area from each T2-weighted image was marked manually and calculated using the software program Osiris (University of Geneva). Relative infarct volume (RIV) was normalized as described by Neumann-Haefelin et al. (2000), using an equation RIV = (LT - (RT - RI)) × d, where LT and RT represented the areas of the left and right hemispheres, respectively, in square millimeters, RI was the infracted area in square millimeters, and d was the slice thickness (1.5 mm). Relative infarct volumes (% HLV) were expressed as a percentage of the right hemispheric volume.
Immunohistochemical analysis and Quantification
To evaluate the proliferation of NSCs, the animals were injected with bromodeoxyuridine (BrdU, 50 mg/kg in saline, i.p.) daily from the transplantation immediately and for subsequent 4 days after transplantation. For the evaluation of the immediate proliferation of NSCs from SVZ, the rats were sacrificed at 2 h after the last BrdU injection at one week (n = 5). Simultaneously, survival or differentiation of newborn cells were evaluated at two weeks, that is, one week after the last BrdU injection (n = 5).
For immunohistochemistry, animals were intracardially perfused with PBS and then fixed with 4% paraformaldehyde (PFA). Brains were embedded in paraffin and sectioned to 5-µm thickness. After boiling in 10 mM sodium citrate (pH 6.0) by microwave for retrieval of antigenicity, the sections were treated with 2 N HCl, and then 0.1 M sodium borate (pH 9.0) and then incubated in PBS containing 1% BSA and 5% normal serum. Then the sections were probed with antibodies recognizing BrdU (mouse, 1:100, Sigma, St Louis, MO, or sheep, 1:100, Abcam, Cambridge, UK), doublecortin (Dcx, 1:100, Santacruz, Santacruz, CA), NeuN (1:100, Chemicon, Temecula, CA). The immunoreactivity was visualized with Alexa Fluor 488- or -594-conjugated anti-IgG secondary antibodies (Molecular Probes, Eugene, OR) and counterstained with bis-benzamide (Molecular Probes). In the case of staining for ischemic cell death, the sections were subjected to In Situ Cell Death Detection kit from Roche Diagnostic GmbH (Manheim, Germany) following to manufacture's protocol. Fluorescent images were acquired using a Zeiss LSM510 confocal microscope (Zeiss, Germany). To identify mitotic cells, the sections were boiled in 10 mM sodium citrate (pH 6.0), and then treated with 0.3% of H2O2. After blocking with normal serum, the sections were probed with anti-Ki-67 antibody (Chemicon) at 4℃ for overnight. The antibody reaction was visualized using an ABC kit (Vector Laboratories, Burlingame, CA). Sections were examined with a Zeiss Axiophot microscope (Zeiss).
Quantification
To quantify the number of immunoreactive cells, three representative sections from each animal (n = 5) were analyzed. The numbers of BrdU+, Dcx+cells, NeuN+ cells, TUNEL+ cells, or Ki-67+ were blindly counted within 0.25 mm2 of subven tricular zone (SVZ), ischemic cortical boundary (ICB), and ischemic striatal boundary (ISB) using NIH image software, Image J. Briefly, after the fluorescent or bright field images were imported to Image J software, the immunoreactive cells of interest were manually marked and calculated with Image J.
The numbers of three sections from each animal were averaged, and the data from 5 animals of each experimental group were presented as means ± SD. Results were analyzed using one-way or repeated measures ANOVA with independent variables of treatment groups and days of testing, followed by Scheff's post-hoc test for multiple comparisons at each measurement day. The level of statistical significance was set at P < 0.05.
Results
Therapeutic effects of MSCs in a rat stroke model
MSCs were isolated from the bone marrow. The MSCs expanded in vitro showed fibroblast-like morphology with spindle shaped body and their multiple potentials to differentiate into adipocytes, osteocytes, and chondrocytes were verified as previously described (Pittenger et al., 1999) (Figure 1).
Differentiation of expanded MSCs into mesenchymal lineage cells in vitro. MSCs from the 8th passage were induced to differentiate into mesenchymal lineage cells as described in the 'Materials and Methods'. (A) Phase-contrast picture of MSCs culture. (B) Adipogenesis was visualized by staining of lipid vacuoles with oil-red O. (C) Chondrogenesis was demonstrated with increased proteoglycan rich extracellular matrix by alcian blue staining. (D) Osteogenic differentiation of MSCs was demonstrated by the increased alkaline phosphatase activity. Scale bars, 100 µm.
To evaluate the therapeutic effects of MSCs in our experimental condition, 3 days after inducing brain ischemia with middle cerebral artery occlusion (MCAo) for 2 h, 1.0 × 106 of MSCs were transplanted into 2 sites of ischemic boundary zone (the dorsolateral striatum and the cortex). Motor functions of the animals were evaluated weekly by rotarod and adhesive removal test. The control animals that received PBS showed spontaneous recovery for the first week to a minimal extent and reached to plateau thereafter (Figure 2A, B, open circles). The animals with MSCs showed higher recovery than the control group in the rotarod and adhesive removal tests (Figure 2A, B, closed circles). The MSC group showed 1.5 fold higher scores in rotarod test (48 ± 7.7 versus 32 ± 6.5%) or 1.9 fold lower scores in adhesive removal test (100 ± 12.5 versus 192 ± 37.7 s) on day 28.
Transplantation of MSCs ameliorates the behavioral impairments and reduces infarct volume in stroke model of rats. Behavioral performance in the rotarod (A) and adhesive removal (B) tests of PBS (open circles) or MSCs (closed circles) treated animals from 1 to 28 days after ischemia. (C) Brain infarct volumes of eight animals in each group were measured using MRI from 2 to 28 days after stroke. (D) Relative infarct volume of PBS (open bars) or MSCs (closed bars)-treated animals are presented as the mean ± S.D. Statistically significant differences between the MSCs group with PBS group were determined by ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001). Tx indicates the time point of transplantation.
The brain structure was monitored by magnetic resonance imaging (MRI) over the 28 days of experimental period. The hyper-intense areas in T2-weighted images over the central 8 images (1.5 mm thick) were obtained to measure the infarct volume (Figure 2C, D). The original infarct volume was around 57% of the intact contralateral hemisphere. In both the control or MSC groups, the infarct volume was spontaneously decreased to 31-34% for the first 2 weeks. Thereafter the control group ceased to recover whereas the infarct of the MSC group continued to decrease (26 ± 3.9% on day 28). These data indicate that the higher motor functions observed in the MSCs-injected group correlated to less ischemic damage and higher tissue integrity in the host brain, which is evident after 2 weeks.
Enhanced neurogenesis by MSCs
Since the beneficial effects of MSCs were evident in delayed period after inflammatory responses subsided, we investigated the contribution of newly generated neurons were enhanced by transplantation of MSCs. We labeled the proliferating NSCs by daily injecting BrdU for 5 days starting right after transplantation till sacrificing on day 7. New born cells generated from the SVZ were identified by the immunoreactivity to BrdU and/or Dcx. Dcx is transiently expressed in newly formed, migrating neuroblasts and often used to trace the nascent cells.
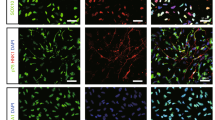
One week after MCAo, most BrdU+, Dcx+ were found within or adjacent to SVZ. The number of BrdU+, Dcx+ cells was robustly increased following transplantation of MSCs by 1.8 fold in SVZ1 and 2.4 fold in SVZ2 (Figure 3B, compare 26 ± 1.3 cells in SVZ1 or 16 ± 6.1 cells in SVZ2 of the control versus 46 ± 7.9 cells in SVZ1 or 38 ± 9.2 cells in SVZ2 of the MSC-group). Since it was shown that the apoptotic cells uptake BrdU in the hypoxic ischemic brain, although the BrdU+ cells are not restricted to the SVZ but rather dispersed in the infarct region (Kuan et al., 2004). Therefore, we further verified proliferation of NSCs resident in SVZ using an anti-Ki67 antibody. Ki67 is a nuclear protein that is expressed in mitotic phases of the cell cycle (Scholzen and Gerdes, 2000). The number of Ki-67+ cells was significantly increased by 2.5 fold in SVZ in MSC-group compared to PBS control (Figure 4, compare 37 ± 15.3 of control versus 93 ± 25.2 of MSC-group). The data indicate that MSCs increased proliferation of resident NSCs.
Endogenous neurogenesis induced by MSCs. Proliferation of endogenous NSCs and subsequent migration was demonstrated by double staining of BrdU with Dcx at 1 week and 2 weeks after MCAo. (A) Schematic illustration of region of investigated was shown (1, subventricular zone1, SVZ1; 2, subventricular zone2, SVZ2; 3, ischemic cortical boundary, ICB; 4, ischemic striatal boundary, ISB). (C, D) Proliferation of NSCs and subsequent migration of newly generated cells were detected by double staining for BrdU (green) and Dcx (red) in SVZ1 and SVZ2 at 1 week; or ICB and ISB at 2 weeks following transplantation of PBS or MSCs. (B) The numbers of BrdU+, Dcx+ cells in 0.25 mm2 of two regions (SVZ1 and SVZ2) indicated in (A) at 1 week or ICB and ISB at 2 weeks were quantified as described in the Methods. (E) Neuronal differentiation of newborn cells was demonstrated by double immunoreactivity to BrdU and NeuN in ICB at 2 weeks after MCAo. Orthographic images of the inset were acquired by higher magnified z-section. (F) Quantitative analysis of BrdU+ cells co-stained with NeuN in 0.25 mm2 of ICB were carried out. The data are presented as the mean numbers of positive cells ± S.D. (ANOVA, *p < 0.05, **p < 0.01). Arrows indicate the BrdU+ cells co-stained with Dcx (C, D) or NeuN (E); Arrowhead indicates BrdU+ cells that were not colocalized with Dcx or NeuN. Scale bars, 10 µm.
MSCs increase mitotic cell division from NSCs. Proliferation of NSCs resident in SVZ was demonstrated by immunoreactivity of Ki-67 at 1 week after MCAo. (A) Ki-67+ (brown) cells indicate the progenitor cells undergoing mitosis. (B) The numbers of Ki-67+ cells in 0.25 mm2 of SVZ were quantified as described in the Methods. The data are presented as the mean numbers of positive cells ± S.D. (ANOVA, **p < 0.01). Scale bars, 50 µm.
In the control group, the BrdU+ cells exhibited condensed, irregular nuclei with rare Dcx expression in the cytosol (Figure 3C), suggesting that most cells undergo apoptotic cell death (see Figure 5). In contrast, the BrdU+ cells of the MSC-group exhibited relatively homogenous oval shaped nuclei and expressed Dcx in the cytosol. Two weeks after MCAo, BrdU+ cells migrated away from SVZ to ischemic territory and widely distributed. Therefore, we measured the number of BrdU+, Dcx+ cells in remote areas near to ischemic territory such as ischemic cortical boundary (ICB) and ischemic striatal boundary (ISB) (Figure 3A). In the control group, most of BrdU+ cells were devoid of Dcx in ICB as well as ISB. Interestingly, BrdU+, Dcx+ cells were found more often by 3.1 fold in ICB and by 2.2 fold in ISB of the MSC-group compared to the control (Figure 3D). These BrdU+, Dcx+ cells showed mature neuronal morphology with long processes, suggesting they are migrating neuroblasts. Moreover the BrdU+ cells in the MSC-group expressed NeuN+, a perinuclear neuron-specific protein by 2.3 fold higher than the control group (7 ± 1.1% of NeuN+ cells in the MSC-group versus 3 ± 1.1% of NeuN+ cells in the control, P < 0.05) (Figure 3E, F). The rare expression of NeuN in the BrdU+ cells further confirms that new born cells in the control animals failed to differentiate into neuronal cells but underwent apoptosis (see below).
MSCs protect newborn cells from the ischemic environment. Prolonged survival of newborn cells was demonstrated by immunoreactivity to BrdU and TUNEL 2 weeks after MCAo. (A) The TUNEL+ (green) and BrdU+ (red) cells indicate the new born cells undergoing apoptotic cell death. Please note that most BrdU+ cells are TUNEL+ in the control animals. (B) Quantitative analysis of BrdU+ cells co-stained with TUNEL in 0.25 mm2 of ICB and ISB were carried out as described in the Methods. The data are presented as the mean numbers of positive cells ± S.D. (ANOVA, *p < 0.05). Arrows indicate BrdU+ cells colocalized with TUNEL; Arrowheads TUNEL+ cells devoid of BrdU in cortex or striatum. Scale bars, 10 µm.
Since the control animals had tendency to have condensed nuclei in BrdU+ cells in greater extent, we investigated whether the newly generated BrdU+ cells might undergo apoptosis while they migrated into ischemic territory (Figure 5). Two weeks after the ischemic injury, 19 ± 4.6% and 14 ± 3.3% of BrdU+ cells were TUNEL+ in ICB and ISB of the control animals, respectively. In the MSC-group, the number of TUNEL+ cells among BrdU+ cells was reduced to 14 ± 2.8% in ICB and more dramatically to 7 ± 3.2% in ISB. Taken together, these data indicate that MSCs not only promote neurogenesis in the SVZ but also protect newborn neuroblasts which migrate toward ischemic parenchyma.
Discussion
In the present study, we demonstrate that transplantation of MSCs enhanced functional recovery, which correlate with increment both in proliferation of endogenous NSCs in the SVZ and in the survival of newborn neuroblasts in the pathological postischemic brain.
The adult mammalian SVZ contains stem cells that slowly proliferate and give rise to Dcx+ neuroblasts. Dcx+ cells migrate as a network of tangentially oriented chains and reach the olfactory bulb (Doetsch et al., 1997, 1999).
After the ischemic injury, the SVZ is expanded (Parent et al., 2002) where proliferation of NSCs is accelerated during first two weeks compared to the normal brain (Zhang et al., 2001a; Arvidsson et al., 2002; Li et al., 2002; Takasawa et al., 2002). The newly generated Dcx+ neuroblasts migrate toward the ischemic territory which is mediated by signaling pathways involving stromal cell-derived factor 1α (SDF-1α) and its receptor CXCR4 (Thored et al., 2006). However, the majority of newborn cells unfortunately die while they migrate toward ischemic parenchyma via caspase-dependent apoptotic processes (Arvidsson et al., 2002; Zhang et al., 2006). This notion is consistent with our results that the PBS-treated control animals showed minimal recovery in motor functions and infarct volumes, and then reached a plateau in two weeks after the stroke.
Our study shows that transplantation of MSCs yields higher numbers of BrdU+, Dcx+ cells in the SVZ one week after ischemic injury compared to the control group, and in ICB and ISB two weeks later. These cells tangentially migrate from the SVZ toward the ischemic territory and disperse widely in ICB and ISB. Therefore, the densities of BrdU+, Dcx+ cells per 0.25 mm2 in ICB and ISB are generally lower at 2 weeks than those in the SVZ at 1 week (Figure 3). Importantly, MSCs increase the number of BrdU+, Dcx+ cells compared to the PBS-injected control group. Our result with MSCs is consistent with previous studies that MSCs produce bioactive cytokines that promote neurogenesis of resident stem cells in the SVZ (Jin et al., 2001; Zhang et al., 2001b).
Earlier studies initially reported that the exposure of MSCs to certain chemicals caused neuron-like morphological changes in vitro culture (Woodbury et al., 2000; Zhao et al., 2002; Chen et al., 2003a). However, it is now believed that rapid morphological change to neuron-like cells is due to immediate cytoskeletal rearrangement but not due to the genuine acquisition of neuronal cell fates (Lu et al., 2004; Neuhuber et al., 2004). In addition, the neuron-like phenotypes of MSCs after transplanted in the brain may be a misinterpretation of the cell-to-cell fusion or reuptake of donor antigen following the ischemic damage (Burns et al., 2006; Coyne et al., 2006). Indeed, we as well as others could not detect expression of NeuN or Dcx in MSCs in vivo (data not shown), indicating that the BrdU+, Dcx+ cells in ICB or ISB are derived from the host NSCs and the numbers of these BrdU+, Dcx+ neuroblasts are increased in response to bioactive factors secreted by transplanted MSCs.
MSCs are known to secrete the many kinds of tropic factors including brain-derived neurotrophic factor (BDNF), VEGF, and nerve growth factor (NGF) (Labouyrie et al., 1999; Kinnaird et al., 2004; Mahmood et al., 2004). Direct infusion of these tropic factors into damaged brain prevents neuronal cell death (Kromer 1987; Hayashi et al., 1998). Therefore, it is very presumable that transplantation of MSCs reduces the number of TUNEL+ cells through via activating signaling pathways involving these trophic factors (Chen et al., 2003a; Kurozumi et al., 2004). Interestingly, the protective effects of MSCs are not limited to pre-existing resident cells but further expanded to newly generated neuroblasts (Figure 5). Taken together, MSCs promote the neurogenesis through dual modes by accelerating proliferation of NSCs in the SVZ as well as by protecting the new born cells from the pathogenic environment while they migrate toward the ischemic territory.
MSCs can be isolated from various tissues including bone marrow, adipose tissue, umbilical cord blood (Wagner et al., 2005; Kern et al., 2006). In those tissues, MSCs act as supporting cells to facilitate proper functions of resident progenitor cells. For example, MSCs in bone marrow regulate the survival, self-renewal, migration, and differentiation of hematopoietic stem cells (HSCs) through several mechanisms, including cell contact interactions or the production of growth factors, chemokines, and extracellular matrix molecules (He et al., 2007). These paracrine functions of MSCs improve microenvironment more favorable to host cells and enhance the recovery when transplanted to animals with non-neurological as well as neurological dysfunctions.
The present study verifies previous findings that therapeutic effects of MSCs are associated with the acceleration of proliferation of NSCs resident in the SVZ, but also firstly reveals that the beneficial effects of MSCs are exerted on newborn neuroblasts. Promotion of neurogenesis by MSCs through dual mechanisms may provide a scientific basis for the potential use of these autograftable cells as a therapeutic tool for the treatment of neurological dysfunctions.
Abbreviations
- BrdU:
-
bromodeoxyuridine
- Dcx:
-
doublecortin
- ICB:
-
ischemic cortical boundary
- ISB:
-
ischemic striatal boundary
- MCAo:
-
middle cerebral artery occlusion
- mNSS:
-
modified neurological severity score
- MSC:
-
mesenchymal stem cell
- NeuN:
-
neuronal nuclei
- NSC:
-
neural stem cell
- PFA:
-
paraformaldehyde
- SVZ:
-
subventricular zone
References
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O . Neuronal replacement from endogenous precursors in the adult brain after stroke . Nat MeCollind 2002 ; 8 : 963 - 970
Burns TC, Ortiz-Gonzalez XR, Gutierrez-Perez M, Keene CD, Sharda R, Demorest ZL, Jiang Y, Nelson-Holte M, Soriano M, Nakagawa Y, Luquin MR, Garcia-Verdugo JM, Prosper F, Low WC, Verfaillie CM . Thymidine analogs are transferred from prelabeled donor to host cells in the central nervous system after transplantation: a word of caution . Stem Cells 2006 ; 24 : 1121 - 1127
Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M . Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat . J Neurosci Res 2003a ; 73 : 778 - 786
Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M . Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke . Ann Neurol 2003b ; 53 : 743 - 751
Coyne TM, Marcus AJ, Woodbury D, Black IB . Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia . Stem Cells 2006 ; 24 : 2483 - 2492
Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A . Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain . J Neurosci 1997 ; 17 : 5046 - 5061
Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A . Subventricular zone astrocytes are neural stem cells in the adult mammalian brain . Cell 1999 ; 97 : 703 - 716
Fouillard L, Labopin M, Gorin NC, Polge E, Prentice HG, Meloni G, Reiffers J, Pigneux A, Willemze R, Schattenberg A, Sica S, Lagrange M, Fenneteau O, Perot C, Frassoni F . Hematopoietic stem cell transplantation for de novo erythroleukemia: a study of the European Group for Blood and Marrow Transplantation (EBMT) . Blood 2002 ; 100 : 3135 - 3140
Hayashi T, Abe K, Itoyama Y . Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia . J Cereb Blood Flow Metab 1998 ; 18 : 887 - 895
Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, Takahashi J, Hashimoto N, Nozaki K . Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain . J Cereb Blood Flow Metab 2006 ; 26 : 906 - 914
He Q, Wan C, Li G . Concise review: multipotent mesenchymal stromal cells in blood . Stem Cells 2007 ; 25 : 69 - 77
Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA . Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat . Proc Natl Acad Sci USA 2001 ; 98 : 4710 - 4715
Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK . Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex . Proc Natl Acad Sci USA 2004 ; 101 : 11839 - 11844
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K . Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue . Stem Cells 2006 ; 24 : 1294 - 1301
Kim SS, Choi JM, Kim JW, Ham DS, Ghil SH, Kim MK, Kim-Kwon Y, Hong SY, Ahn SC, Kim SU, Lee YD, Suh-Kim H . cAMP induces neuronal differentiation of mesenchymal stem cells via activation of extracellular signal-regulated kinase/MAPK . Neuroreport 2005 ; 16 : 1357 - 1361
Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE . Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms . Circulation 2004 ; 109 : 1543 - 1549
Kohyama J, Abe H, Shimazaki T, Koizumi A, Nakashima K, Gojo S, Taga T, Okano H, Hata J, Umezawa A . Brain from bone: efficient "meta-differentiation" of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent . Differentiation 2001 ; 68 : 235 - 244
Kromer LF . Nerve growth factor treatment after brain injury prevents neuronal death . Science 1987 ; 235 : 214 - 216
Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, Sharp FR, Rakic P . Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain . J Neurosci 2004 ; 24 : 10763 - 10772
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H . BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model . Mol Ther 2004 ; 9 : 189 - 197
Kwak DH, Yu K, Kim SM, Lee DH, Kim SM, Jung JU, Seo JW, Kim N, Lee S, Jung KY, You HK, Kim HA, Choo YK . Dynamic changes of gangliosides expression during the differentiation of embryonic and mesenchymal stem cells into neural cells . Exp Mol Med 2006 ; 38 : 668 - 676
Labouyrie E, Dubus P, Groppi A, Mahon FX, Ferrer J, Parrens M, Reiffers J, de Mascarel A, Merlio JP . Expression of neurotrophins and their receptors in human bone marrow . Am J Pathol 1999 ; 154 : 405 - 415
Lee KJ, Kim SJ, Kim SW, Choi SH, Shin YC, Park SH, Moon BH, Cho E, Lee MS, Choi SH, Chun BG, Shin KH . Chronic mild stress decreases survival, but not proliferation, of new-born cells in adult rat hippocampus . Exp Mol Med 2006 ; 38 : 44 - 54
Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K . Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy . Clin Pharmacol Ther 2008 ; 83 : 723 - 730
Li Z, Kato T, Kawagishi K, Fukushima N, Yokouchi K, Moriizumi T . Cell dynamics of calretinin-immunoreactive neurons in the rostral migratory stream after ibotenate-induced lesions in the forebrain . Neurosci Res 2002 ; 42 : 123 - 132
Longa EZ, Weinstein PR, Carlson S, Cummins R . Reversible middle cerebral artery occlusion without craniectomy in rats . Stroke 1989 ; 20 : 84 - 91
Lu P, Blesch A, Tuszynski MH . Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact ? J Neurosci Res 2004 ; 77 : 174 - 191
Mahmood A, Lu D, Chopp M . Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury . J Neurotrauma 2004 ; 21 : 33 - 39
Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ . Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice . Proc Natl Acad Sci USA 2005 ; 102 : 18171 - 18176
Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fischer I . Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype . J Neurosci Res 2004 ; 77 : 192 - 204
Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, Moseley ME . Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation . Stroke 2000 ; 31 : 1965 - 1972
Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM . Rat forebrain neurogenesis and striatal neuron replacement after focal stroke . Ann Neurol 2002 ; 52 : 802 - 813
Park KI, Teng YD, Snyder EY . The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue . Nat Biotechnol 2002 ; 20 : 1111 - 1117
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR . Multilineage potential of adult human mesenchymal stem cells . Science 1999 ; 284 : 143 - 147
Scholzen T, Gerdes J . The Ki-67 protein: from the known and the unknown . J Cell Physiol 2000 ; 182 : 311 - 322
Silani V, Cova L, Corbo M, Ciammola A, Polli E . Stem-cell therapy for amyotrophic lateral sclerosis . Lancet 2004 ; 364 : 200 - 202
Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA . VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia . J Clin Invest 2003 ; 111 : 1843 - 1851
Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K, Ohstuki T, Miyata T, Okano H, Hori M, Matsumoto M . Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats . J Cereb Blood Flow Metab 2002 ; 22 : 299 - 307
Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY . Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells . Proc Natl Acad Sci USA 2002 ; 99 : 3024 - 3029
Teramoto T, Qiu J, Plumier JC, Moskowitz MA . EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia . J Clin Invest 2003 ; 111 : 1125 - 1132
Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O . Persistent production of neurons from adult brain stem cells during recovery after stroke . Stem Cells 2006 ; 24 : 739 - 747
Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD . Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood . Exp Hematol 2005 ; 33 : 1402 - 1416
Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, Chopp M . MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture . Hematology 2002 ; 7 : 113 - 117
Wang L, Zhang Z, Wang Y, Zhang R, Chopp M . Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats . Stroke 2004 ; 35 : 1732 - 1737
Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H . Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial . Lancet 2004 ; 364 : 141 - 148
Woodbury D, Schwarz EJ, Prockop DJ, Black IB . Adult rat and human bone marrow stromal cells differentiate into neurons . J Neurosci Res 2000 ; 61 : 364 - 370
Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M . A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats . Ann Neurol 2001 ; 50 : 602 - 611
Zhang R, Xue YY, Lu SD, Wang Y, Zhang LM, Huang YL, Signore AP, Chen J, Sun FY . Bcl-2 enhances neurogenesis and inhibits apoptosis of newborn neurons in adult rat brain following a transient middle cerebral artery occlusion . Neurobiol Dis 2006 ; 24 : 345 - 356
Zhang RL, Zhang ZG, Zhang L, Chopp M . Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia . Neuroscience 2001 ; 105 : 33 - 41
Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC . Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats . Exp Neurol 2002 ; 174 : 11 - 20
Acknowledgements
This study was supported by grants from the Korea Health 21R&D Project (0405-DB01-0104-0006), Ministry of Health and Welfare and Brain Research Center of the 21st Century Frontier Research Program (M103KV010008-07K2201-00810) to HSK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yoo, SW., Kim, SS., Lee, SY. et al. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med 40, 387–397 (2008). https://doi.org/10.3858/emm.2008.40.4.387
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2008.40.4.387
Keywords
This article is cited by
-
MesenchymAl stromal cells for Traumatic bRain Injury (MATRIx): a study protocol for a multicenter, double-blind, randomised, placebo-controlled phase II trial
Intensive Care Medicine Experimental (2023)
-
Intracerebral Transplantation of Autologous Mesenchymal Stem Cells Improves Functional Recovery in a Rat Model of Chronic Ischemic Stroke
Translational Stroke Research (2023)
-
Potential of stem cell therapy in intracerebral hemorrhage
Molecular Biology Reports (2020)
-
Immunomodulatory effect of CD200-positive human placenta-derived stem cells in the early phase of stroke
Experimental & Molecular Medicine (2018)
-
Human placenta-derived mesenchymal stem cells loaded on linear ordered collagen scaffold improves functional recovery after completely transected spinal cord injury in canine
Science China Life Sciences (2018)