Abstract
The increased knowledge of genetics has raised new questions, and confusion has been growing about the evaluation of the results of recent research and the role of geneticists in the genomic medicine. If we focus on transgenerational and developmental aspects of diseases, the answers might be more evident.
Similar content being viewed by others
Main
The completion of the human genome project has increased the understanding of the role of genes in rare and common diseases. Although the primary driving factor in the development of genetic knowledge is undoubtedly research, the main challenge is to ensure that scientific results are adequately translated into validated diagnostic procedures and quality health care. Recent research reports and editorials of leading scientific journals have indicated that many geneticists – researchers and clinical geneticists – are disappointed by the limited results of genome-wide association studies (GWAS) considered for a while as ‘the’ approach to identify disease-related genes.1, 2, 3 Although the achievements of the human genome project have been widely acclaimed and its impact on new insights in the function of our genome is unquestionable, confusion has been growing about the role of the new genetics or genomics in medical practice. Uncertainty is also emerging about who will deliver the necessary services in the future ‘genomic medicine’ and what the precise role of the geneticist might be in this.4
Looking for answers to these uncertainties, it is necessary to examine what is really new in genomic medicine and whether the distinction between genetics and genomics is helpful in considering who does what in medicine.
Guttmacher and Collins5 in their influential survey wrote: ‘If genetics has been misunderstood, genomics is even more mysterious – what, exactly, is the difference?’ They went on: ‘Genetics is the study of single genes and their effects. Genomics is the study not just of single genes, but of the functions and interactions of all the genes in the genome. Genomics has a broader and more ambitious reach than does genetics … to the entire genome and applies to common conditions’.
In 2006, Charles Epstein6 wrote: ‘The term “medical genetics” has been variously defined as the science of human biological variation as it relates to health and disease; the study of the etiology, pathogenesis, and natural history of diseases, and disorders that are at least partially genetic in origin’. He continued: ‘…genomic medicine is what has been variously described as preventive intervention, individualized treatment, prospective medicine, or personalized medicine’.
We do not want to deny that differences exist between ‘classical genetics’ and ‘-omics’ in the way that the two terms are usually used in research or in explaining and understanding living structures. Still, a forced distinction of genomics from genetics is difficult and even unnecessary. Instead of trying to define firmly what genetics is and what is genomics, we recommend to focus on the transgenerational and developmental aspects of diseases.
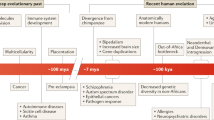
Indeed, genetics focuses on (1) how the genetic information and the traits under genetic control are transmitted vertically through generations (transgenerational aspect), and (2) how the genome, assembled in the zygote, contributes to the development of a new individual and how this becomes gradually manifest during the life span of the individual (developmental aspect). Although these two aspects are implicitly present in the various definitions, to our knowledge no explicit statement exists that points at this distinction. In the transgenerational aspect, the approach is germ cell focused, risk assessment of inheritance is essential, reproductive fitness is important, and gene–environment interactions are of limited significance. In the developmental aspect, the focus is on somatic cells, susceptibility or predisposition for late-onset diseases is important, gene–environment interactions are significant (epigenetics), gene therapy to correct gene mutations is acceptable, and the individual approach (personalized medicine) is a real promise.
When the contribution of recent research results is evaluated for these two aspects, it becomes clear why geneticists can be disappointed about the GWAS results.
Ten years ago, it was predicted that the advances in genome research would revolutionize our understanding of the inheritance not only of single-gene and chromosomal disorders, but also of common or complex diseases. Although the GWAS promised to identify many of the genes involved, the individual and cumulative effects found are small and nowhere close enough to explaining earlier estimates of heritability (missing heritability).2 GWAS are turning up dozens of DNA sequence variations that increase risk only modestly. A possible explanation for ‘missing heritability’, such as sequence variations in single genes, copy-number variations, epigenetics, epistasis, which are only poorly captured by GWAS, could explain the limitation of GWAS.7 It is clear that the GWAS results have thus far contributed only in a limited manner to the understanding of the transgenerational aspects of common diseases.
In the assessment of the reproductive risk that, in the transgenerational aspect at least, is one of the main clinical applications of genetic knowledge, it does make a great difference whether the disease is determined by one, a few, or many genes. Compared to common disorders, risk assessment for single-gene disorders is easier not only because of our better knowledge of the genomic changes responsible for monogenic diseases but also because of our understanding of the Mendelian laws and their exceptions. Nevertheless, recent research has significantly increased our understanding of many single-gene disorders, which has facilitated clinical diagnosis, including prenatal and preimplantation tests, disease prediction, prevention, resulting in more efficient reproductive risk assessment.8, 9 Accordingly, the impact of genomic research on health service delivery has resulted in significant improvement for single-gene disorders even in the transgenerational aspect.
The results of GWAS on the other hand have nevertheless benefited our understanding of the developmental aspects of common diseases.10 Genes have been identified that have a role in the pathogenesis of different disorders. Genomics has uncovered disease mechanisms by revealing vast networks of interacting genes, by examining how variations in the genotype and variation in gene expression are related (integrated genomics).11 It can be hoped that in time, new technologies will allow researchers to examine genetic mutations at the functional level and to unravel the biology of systems, epigenomic and pleiotropic effects, the role of environmental factors, nutrition or personal behavior, and their role in causing diseases.12 The real promise is to elucidate disease mechanisms, a knowledge that will have, at the end, great impact on the individuals’ health and on the population at large.
The separation of the genetic approach into the two suggested aspects may help in clarifying the role of health professionals in the future genomic medicine. Genomic medicine has transcended the current boundaries of medical genetics and will soon be applicable to the health care of the many rather than just a few. This will need the contribution of professionals of many different fields. Different types of health professionals, using their different knowledge bases and talents, will contribute to the correct management of diseases.4, 13 We have therefore now truly reached the point of redefining the roles of the geneticist. ‘If we genetic specialists do not embrace change, our role both in shaping and providing genomic medicine will be marginal. If we welcome and help to shape the change, we can lead rewarding professional lives while making significant contributions to the health of all.’14
In service delivery a leading role for geneticists must be maintained in understanding both the transgenerational and developmental aspects, but the need for certain genetic competences of nongenetic health professionals will be required for the developmental aspects. Knowledge of the natural history of neurological disorders, for instance, understanding how the genetic program is unraveled during the life span of the patients, will allow the neurologists to communicate information about the genetic components of the disease in an understandable way in a specialty-focused view. This will help patients to make informed decisions about their care, to be familiar with the uses and limitations of genetic testing, and to be able to use genetic testing appropriately, competencies that are more understandable through the developmental aspects of the disease. In contrast, the transgenerational aspects, important for determining the risk of occurrence or recurrence of a disease, to provide genetic information that helps individuals or couples make informed reproductive decisions, help individuals and families to understand the information provided during genetic counseling, or determine the need for and use of genetic tests in disease prediction should be kept in the expertise of the genetic specialists.
To help European Union (EU) Member States in deciding what education and training programs should be offered to prepare the medical profession and allied health professionals to provide optimal services to the population, an expert group of the EuroGentest NoE has prepared a document that delineates the core competences for genetic service delivery in Europe for different types of health professionals including medical doctors, laboratory scientists, nurses, specialists in genetics, etc ((http://www.eurogentest.org/professionals/education/). Also, the Public Health Genomics European Network (PHGEN) is currently developing ‘European Best Practice Guidelines for Quality Assurance, Provision and Use of Genome-based Information and Technologies’, which should assist the EU Member States in policymaking (http://www.phgen.eu).
In conclusion, it is clear that the unique expertise of the geneticists will not only continue but will even increase to be essential in the management of rare and common diseases. As the ongoing genomic research increases our understanding of how diseases are caused and progress, one will be able to more clearly distinguish the transgenerational from the developmental aspects of diseases. The role of clinical geneticists in the future should be shaped accordingly while close collaboration with other health professionals with expertise in the developmental aspects will become essential to provide patients with optimal and quality guaranteed management options.
References
Couzin J : With new disease genes, a bounty of questions. Science 2008; 319: 1754–1755.
Editorials: Your life in your hands: instructions for the personal genome age. Nature 2008; 456: 1–3.
Hayden EC : Genomics shifts focus to rare diseases. Nature 2009; 461: 458–459.
Scheuner MT : Delivery of genomic medicine for common chronic adult diseases. JAMA 2008; 299: 1320–1334.
Guttmacher AE, Collins FS : Genomic medicine – a primer. N Eng J Med 2002; 347: 1512–1520.
Epstein CJ : Medical genetics in the genomic medicine of the 21st Century. Am J Hum Genet 2006; 79: 434–438.
Manolio TA, Collins FS, Cox NJ et al: Finding the missing heritability of complex diseases. Nature 2009; 461: 747–753.
Ropers H-H : New perspectives for the elucidation of genetic disorders. Am J Hum Genet 2007; 81: 199–207.
Biesecker LG : Exome sequencing makes medical genomics a reality. Nat Genet 2010; 42: 13–14.
Chi KE : Human genetics: hit or miss? Nature 2009; 461: 712–714.
Nelson B : Personal genomes: a disruptive personality, disruptive. Nature 2008; 456: 26–28.
Brand A, Brand H, Schulte T : The impact of genetics and genomics on public health. Eur J Hum Genet 2008; 16: 5–13.
Ginsburg GS : Grand challenges’ in the translation of genomics to human health. Eur J Hum Genet 2008; 16: 873–874.
Guttmacher AE, Jenkins J, Uhlmann WR : Genomic medicine: who will practice it? A call to open arms. Am J Med Genet 2001; 106: 216–222.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kosztolányi, G., Cassiman, JJ. The medical geneticist as expert in the transgenerational and developmental aspects of diseases. Eur J Hum Genet 18, 1075–1076 (2010). https://doi.org/10.1038/ejhg.2010.100
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2010.100
Keywords
This article is cited by
-
Hypothesis: epigenetic effects will require a review of the genetics of child development
Journal of Community Genetics (2011)
-
Some considerations about a report on ‘Public health in an era of genomic-based and personalized medicine’ from the Public Health Foundation, Cambridge
Journal of Community Genetics (2011)