Abstract
A high homocysteine, low folate phenotype is a feature of many diseases. The effect of the cystathionine β-synthase (CBS) 844ins68 polymorphism on homocysteine and folate concentrations was examined alone and in the context of the 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism in a Northwestern European male population. The MTHFR 677TT genotype is known to be associated with increased homocysteine and decreased folate relative to CT heterozygotes and CC homozygotes in this and other populations. MTHFR 677TT homozygotes who were also CBS 844ins68 carriers had homocysteine and folate concentrations similar to those of individuals with the MTHFR 677CT and CC genotypes. Homocysteine levels in MTHFR 677TT subjects carrying the CBS 844ins68 allele were 24.1% lower than in non-carriers (6.66 vs 8.77 μmol/l, P=0.045), and serum folate levels were 27.7% higher (11.16 vs 8.74 nmol/l, P=0.034). These findings suggest that the CBS 844ins68 allele ‘normalizes’ homocysteine and folate levels in MTHFR 677TT individuals.
Similar content being viewed by others
Introduction
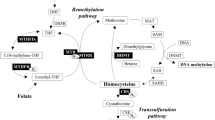
The folate/homocysteine metabolic pathway provides one-carbon units for important biological functions, such as methylation of DNA and proteins, and support of thymidylate and purine synthesis. A phenotype characterized by high homocysteine and low folate may be pathogenic and has been associated with diverse conditions ranging from cardiovascular diseases1 to birth defects including spina bifida.2 Homocysteine concentrations are influenced by dietary factors such as folate and B vitamins, lifestyle factors such as smoking, and genetic factors such as functional polymorphisms in the enzymes of folate/homocysteine metabolism.3
The key enzyme 5,10-methylenetetrahydrofolate reductase (MTHFR, EC 1.5.1.20) generates 5-methyltetrahydrofolate, the methyl donor that is used for homocysteine remethylation to methionine. Cystathionine β-synthase (CBS, EC 4.2.1.22) is a central enzyme in the transsulfuration pathway that irreversibly metabolizes homocysteine to cystathionine. Individuals homozygous for loss-of-function mutations in either of these genes have homocystinuria, characterized by extremely high homocysteine concentrations. Mildly dysfunctional variants of MTHFR have a more modest, but significant, effect on homocysteine concentration and are associated with hyperhomocysteinemia. The most widely studied functional variant is the MTHFR 677C>T (A222V; dbSNP rs1801133) polymorphism, the T allele of which encodes a thermolabile product with mildly impaired activity.4 MTHFR 677TT homozygotes are more likely than those with the CT or CC genotypes to have hyperhomocysteinemia, especially under low folate conditions.5, 6
The CBS 844ins68 allele contains an insertion of 68 bp in exon 8. First reported in a homocystinuric patient by Sebastio et al,7 this polymorphism was initially thought to mandate the use of an insertion-associated premature stop codon in the CBS mRNA leading to the translation of a truncated inactive enzyme. Subsequently Tsai et al8 showed that the 68 bp insertion generates an alternative splice site that permits the elimination of the entire inserted region, thereby allowing the formation of a normal mRNA transcript and a fully functional CBS enzyme. In a Europe-wide study De Stefano et al9 reported that MTHFR 677TT homozygotes who carry a CBS 844ins68 allele had lower homocysteine levels (similar to those observed in MTHFR 677 CT or CC subjects) than noncarriers; however, folate levels were not presented in this report. More recently Dekou et al10 also reported that the CBS 844ins68 allele appears to have a homocysteine lowering effect in MTHFR 677TT homozygotes, but again no data were reported for the effect on folate levels. The study described here was designed to determine whether the CBS 844ins68 allele, alone or in the context of MTHFR 677C>T genotype, has an impact on folate as well as homocysteine concentrations.
Subjects and methods
Subjects (n=614) were drawn from the ‘Industrial Workers Study’ comprising men aged 29–53 years who at the time of recruitment worked for an industrial company in Belfast, Northern Ireland.5, 11 The study was approved by the Research Ethics Committee of the Faculty of Medicine, The Queen's University of Belfast. All subjects provided written informed consent before participation. None of the participants had diabetes and none were taking nutritional supplements. Blood samples were collected from fasting subjects for determination of biochemical parameters and DNA extraction. Total homocysteine concentrations were determined previously5 using an established high-performance liquid chromatography method,12 and serum folate concentrations were determined previously using a commercial kit (ICN Pharmaceuticals).
MTHFR 677C>T genotyping was RFLP based,4 and genotypes for the study population have previously been reported.5 CBS 844ins68 genotypes were obtained using a published PCR-based size difference method.13 Statistical analyses were performed with SAS version 9.1. Even after logarithmic transformation, distributions of homocysteine and serum folate were positively skewed, so all analyses were performed using untransformed data. Hardy–Weinberg equilibrium for the MTHFR 677C>T and CBS 844ins68 genotypes were assessed by the χ2-test. Differences between groups for homocysteine and serum folate were assessed using the Wilcoxon rank-sum or Kruskal–Wallis test.
Results
The MTHFR 677C>T and CBS 844ins68 genotype frequencies were both in Hardy–Weinberg equilibrium (P=0. 923 and P=0.153, respectively). Frequencies of the former (45% CC, 43.6% CT and 11.4% TT) have been previously reported.5 Genotype frequencies for the latter, together with homocysteine and serum folate concentrations are presented in Table 1.
As previously reported,5 MTHFR 677TT homozygotes have markedly higher homocysteine and substantially lower folate concentrations than those with the CT and CC genotypes. As depicted in Figure 1, genotype-specific homocysteine values are 8.55, 7.33, and 6.81 μmol/l, respectively (P<0.0001 by Kruskal–Wallis), and as shown in Figure 2, the genotype-specific serum folate values for these three genotypes are 8.97, 10.93, and 11.50 nmol/l, respectively (P<0.0001 by Kruskal–Wallis).
Median homocysteine levels by cystathionine β-synthase (CBS) 844ins68 genotype within each 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C>T genotype class. Bars represent homocysteine levels in each MTHFR 677C>T genotype class: regardless of CBS 844ins68 genotype, in CBS 844ins68 noncarriers, and in CBS 844ins68 carriers, respectively. The number of subjects in each group is given. †MTHFR 677TT homozygotes have significantly higher homocysteine concentrations than CT heterozygotes and CC homozygotes (P<0.0001 by Kruskal–Wallis).
Median serum folate levels by cystathionine β-synthase (CBS) 844ins68 genotype within each 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C>T genotype class. Bars represent serum folate levels in each MTHFR 677C>T genotype class: regardless of CBS 844ins68 genotype, in CBS 844ins68 noncarriers, and in CBS 844ins68 carriers, respectively. The number of subjects in each group is given. †MTHFR 677TT homozygotes have significantly lower serum folate concentrations than CT heterozygotes and CC homozygotes (P<0.0001 by Kruskal–Wallis).
In the study population as a whole, CBS 844ins68 carriers have marginally lower homocysteine levels and marginally higher folate levels compared to noncarriers, but these modest differences are not statistically significant (Table 1). When considered in the context of each of the MTHFR 677C>T genotypes, CBS 844ins68 carrier status has no significant impact on homocysteine or folate levels in either MTHFR 677CT heterozygotes or CC homozygotes (Figures 1 and 2), but in MTHFR 677TT homozygotes it was associated with 24.1% lower homocysteine (6.66 vs 8.77 μmol/l, P=0.045) and 27.7% higher folate (11.16 vs 8.74 nmol/l, P=0.034). The CBS 844ins68 allele appears to ‘counterbalance’ the homocysteine-raising and folate-lowering effect of the TT genotype such that TT/844ins68 carriers have homocysteine and folate concentrations that are similar to those of MTHFR 677CT heterozygotes or CC homozygotes.
Discussion
The MTHFR 677C>T polymorphism has a major impact on homocysteine levels in populations with a large proportion of individuals with low folate status14 including the cohort reported here. Dekou et al10 and Fredriksen et al15 have both reported that CBS 844ins68 carriers have fasting homocysteine levels that are modestly but significantly lower than those of noncarriers, although others have found no statistically significant difference in homocysteine levels between carriers and noncarriers.16, 17, 18, 19 Interestingly, some studies in which carrier status did not influence fasting homocysteine found that after methionine loading, used experimentally to raise homocysteine concentrations and induce the CBS-mediated transsulfuration pathway, the CBS 844ins68 allele is associated with decreased homocysteine levels.20, 21, 22 Although the exact mechanism by which the 844ins68 allele affects CBS function is currently unknown, it may mandate an increase in CBS activity, possibly via up-regulation of the amount of CBS mRNA or though the action of another functional polymorphism with which it is in linkage disequilibrium.20 Others have reported effects attributable to the 699C>T and 1080C>T polymorphisms; however, these are both silent changes that themselves are unlikely to have a direct effect indicating the possibility of linkage disequilibrium with as yet unknown transcriptional control elements.15, 23
Two reports, a European-wide study9 and a subsequent study from the United Kingdom,10 have suggested that the CBS 844ins68 allele may counterbalance the homocysteine-raising effect of the MTHFR 677TT genotype, although these findings were not replicated in a subsequent UK study.19 Our results, derived from a Northern Irish sample, support the reports that homocysteine levels in MTHFR 677TT homozygotes who carry the CBS 844ins68 allele are significantly lower than in those who do not. In addition, we have made the novel observation that the subset of the MTHFR 677TT genotype class that are CBS 844ins68 carriers (ie 15% of MTHFR 677TT homozygotes) have significantly higher serum folate concentrations than noncarriers. Our results may therefore have important implications for a range of conditions, such as cardiovascular disease and spina bifida in which high homocysteine and low folate each appear to be a contributing feature. The excess risk for such conditions conferred by MTHFR 677TT homozygosity may be negated, at least in part, by CBS 844ins68 carrier status. This hypothesis will be testable in future disease association studies that are large enough to have sufficient power to examine gene–gene interactions.
In conclusion, the CBS 844ins68 allele itself has no statistically significant impact on homocysteine or folate concentrations in a healthy Northwestern European male population. However, it appears to counterbalance the homocysteine-raising and folate-lowering effects of the MTHFR 677TT genotype such that the concentrations of these biochemical variables are essentially the same as those observed in MTHFR 677CT or CC individuals. Thus, the CBS 844ins68 allele may ameliorate the excess risk associated with a high homocysteine and low folate phenotype to which MTHFR 677TT homozygotes would otherwise be predisposed, thereby favorably modifying the risk of pathologies attributable to this MTHFR genotypic class.
References
Refsum H, Ueland PM, Nygard O, Vollset SE : Homocysteine and cardiovascular disease. Annu Rev Med 1998; 49: 31–62.
van der Put NM, van Straaten HW, Trijbels FJ, Blom HJ : Folate, homocysteine and neural tube defects: an overview. Exp Biol Med (Maywood) 2001; 226: 243–270.
Schneede J, Refsum H, Ueland PM : Biological and environmental determinants of plasma homocysteine. Semin Thromb Hemost 2000; 26: 263–279.
Frosst P, Blom HJ, Milos R et al: A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995; 10: 111–113.
Harmon DL, Woodside JV, Yarnell JW et al: The common ‘thermolabile’ variant of methylene tetrahydrofolate reductase is a major determinant of mild hyperhomocysteinaemia. QJM 1996; 89: 571–577.
Jacques PF, Bostom AG, Williams RR et al: Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation 1996; 93: 7–9.
Sebastio G, Sperandeo MP, Panico M, de Franchis R, Kraus JP, Andria G : The molecular basis of homocystinuria due to cystathionine beta-synthase deficiency in Italian families, and report of four novel mutations. Am J Hum Genet 1995; 56: 1324–1333.
Tsai MY, Bignell M, Schwichtenberg K, Hanson NQ : High prevalence of a mutation in the cystathionine beta-synthase gene. Am J Hum Genet 1996; 59: 1262–1267.
De Stefano V, Dekou V, Nicaud V et al: Linkage disequilibrium at the cystathionine beta synthase (CBS) locus and the association between genetic variation at the CBS locus and plasma levels of homocysteine. The Ears II Group. European Atherosclerosis Research Study. Ann Hum Genet 1998; 62: 481–490.
Dekou V, Gudnason V, Hawe E, Miller GJ, Stansbie D, Humphries SE : Gene-environment and gene–gene interaction in the determination of plasma homocysteine levels in healthy middle-aged men. Thromb Haemost 2001; 85: 67–74.
Woodside JV, Yarnell JW, McMaster D et al: Effect of B-group vitamins and antioxidant vitamins on hyperhomocysteinemia: a double-blind, randomized, factorial-design, controlled trial. Am J Clin Nutr 1998; 67: 858–866.
Ubbink JB, van der Merwe A, Delport R et al: The effect of a subnormal vitamin B-6 status on homocysteine metabolism. J Clin Invest 1996; 98: 177–184.
Barbaux S, Kluijtmans LA, Whitehead AS : Accurate and rapid ‘multiplex heteroduplexing’ method for genotyping key enzymes involved in folate/homocysteine metabolism. Clin Chem 2000; 46: 907–912.
Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE : Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci 2001; 22: 195–201.
Fredriksen A, Meyer K, Ueland PM, Vollset SE, Grotmol T, Schneede J : Large-scale population-based metabolic phenotyping of thirteen genetic polymorphisms related to one-carbon metabolism. Hum Mutat 2007; 28: 856–865.
Kluijtmans LA, Boers GH, Trijbels FJ, van Lith-Zanders HM, van den Heuvel LP, Blom HJ : A common 844INS68 insertion variant in the cystathionine beta-synthase gene. Biochem Mol Med 1997; 62: 23–25.
Wang XL, Duarte N, Cai H et al: Relationship between total plasma homocysteine, polymorphisms of homocysteine metabolism related enzymes, risk factors and coronary artery disease in the Australian hospital-based population. Atherosclerosis 1999; 146: 133–140.
Kluijtmans LA, Young IS, Boreham CA et al: Genetic and nutritional factors contributing to hyperhomocysteinemia in young adults. Blood 2003; 101: 2483–2488.
Bowron A, Scott J, Stansbie D : The influence of genetic and environmental factors on plasma homocysteine concentrations in a population at high risk for coronary artery disease. Ann Clin Biochem 2005; 42: 459–462.
Tsai MY, Yang F, Bignell M, Aras O, Hanson NQ : Relation between plasma homocysteine concentration, the 844ins68 variant of the cystathionine beta-synthase gene, and pyridoxal-5′-phosphate concentration. Mol Genet Metab 1999; 67: 352–356.
Tsai MY, Bignell M, Yang F, Welge BG, Graham KJ, Hanson NQ : Polygenic influence on plasma homocysteine: association of two prevalent mutations, the 844ins68 of cystathionine beta-synthase and A(2756)G of methionine synthase, with lowered plasma homocysteine levels. Atherosclerosis 2000; 149: 131–137.
Janosikova B, Pavlikova M, Kocmanova D et al: Genetic variants of homocysteine metabolizing enzymes and the risk of coronary artery disease. Mol Genet Metab 2003; 79: 167–175.
Aras O, Hanson NQ, Yang F, Tsai MY : Influence of 699C → T and 1080C → T polymorphisms of the cystathionine beta-synthase gene on plasma homocysteine levels. Clin Genet 2000; 58: 455–459.
Acknowledgements
This work was supported by National Institutes of Health grants AR47663, CA108862, ES013508, HD039195; PA Department of Health grant 4100038714; the Wellcome Trust, and the British Heart Foundation. Initial funding was obtained through the Institute of Vitamin Research in Berne, Switzerland, courtesy of Professor Fred Gey. Our work was not influenced by these funding agencies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Summers, C., Hammons, A., Mitchell, L. et al. Influence of the cystathionine β-synthase 844ins68 and methylenetetrahydrofolate reductase 677C>T polymorphisms on folate and homocysteine concentrations. Eur J Hum Genet 16, 1010–1013 (2008). https://doi.org/10.1038/ejhg.2008.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2008.69
Keywords
This article is cited by
-
Characterisation of a human liver cystathionine beta synthase mRNA sequence corresponding to the c.[833T>C;844_845ins68] mutation in CBS gene
Molecular and Cellular Biochemistry (2009)