Abstract
The aims of this study were (1) to determine trends in total prevalence and live birth prevalence of Down syndrome, (2) to analyse trends in factors likely to influence this prevalence and (3) to determine 1-year survival in Down syndrome. A retrospective review was made of prospectively collected data on all cases of Down syndrome within a malformation registry born in 1985–2004. Down syndrome affected 1188 pregnancies among 690 215 live births (1.72 per 1000 total births). The proportion increased over 20 years from 1.3 to 2.5 per 1000 total births (P<0.0001). There were 389 terminations for Down syndrome and 51 stillbirths. There were 748 live births with Down syndrome (1.08 per 1000 live births). The live birth prevalence declined in 1985–1994 and increased in 1995–2004 with no overall change. Total live births in the population declined by 20% over 20 years. Mothers delivering at 35 years of age or above increased from 6 to 15%. The uptake of maternal serum screening increased from zero in 1987 to 35% in 1993 but then plateaued. One-year survival of live births with Down syndrome increased, especially in babies with cardiovascular malformations, reaching almost 100%. The prevalence of pregnancies affected by Down syndrome has increased significantly, but there has been no overall change in live birth prevalence. Increasing maternal age and improved survival of children with Down syndrome have offset the effects of prenatal diagnosis followed by the termination of pregnancy and declining general birth rate.
Similar content being viewed by others
Introduction
Down syndrome is the most commonest chromosomal abnormality in live-born infants1, 2 and the most commonest congenital cause of intellectual disability.3 Various screening strategies have been introduced over the years with the aim of offering prospective parents the ability to make informed decisions. The impact of screening has generally been less than that predicted by modelling studies.4, 5
Other temporal trends, particularly changes in birth rate and maternal age profile, are also likely to have an influence on the live birth rate of Down syndrome. Survival of infants with Down syndrome has improved because of better care (especially of cardiovascular malformations) and survival into mid or late adult life is now expected. Few adults with Down syndrome are likely to be capable of unsupported independent existence. Any change in the birth prevalence and survival of Down syndrome will have implications for the provision of long-term care of children and adults with this condition.
Screening and prenatal diagnosis were recommended in the United Kingdom by the Royal College of Obstetrics and Gynaecology in 1993. Screening strategies in the northern region varied during the study period – serum screening was first used in a pilot project in two health districts in 1988 and was introduced across the region in 1991. Maternity units responsible for about 50% of deliveries offered screening to all pregnant women, regardless of age, since 1992. The remaining units offered amniocentesis on an age-related basis. In 1997–2000, two of these units, responsible for about 8% of deliveries, began to offer serum screening to all mothers. Thus, by 2000, serum screening was available to nearly 60% of mothers in this region. In the study area, it would appear that where serum screening for trisomy 21 is offered regardless of age, it is taken up by about 50% of women.
This study was performed to assess trends in the prevalence at live birth of Down syndrome in 1985–2004 in one health region of the United Kingdom. We also looked at trends in survival to 1 year in infants with Down syndrome with and without cardiac defects.
Methods
We based this study on the former Northern Health Region of England. The resident population is around three million with a recent average birth rate of around 35 000 per year. For the years in question (1 January 1985 to 31 December 2004), we obtained details of all live births and live births of infants with Down syndrome confirmed by karyotype (with no upper age limit at diagnosis) from the Northern Congenital Abnormality Survey (NorCAS). NorCAS has collected data on all chromosomal abnormalities and all congenital malformations in this population since 1985.6 NorCAS is a member of the EUROCAT network of population-based registries for surveillance of congenital abnormalities and follows EUROCAT methodology.7 The Northern Genetics Service, Newcastle Hospitals NHS Foundation Trust, provided data on the uptake of maternal serum screening, chorionic villus biopsy and amniocentesis. We obtained details of terminations of pregnancy because of Down syndrome in the region from NorCAS. The Regional Paediatric Cardiology Database provided data on cardiovascular malformations associated with Down syndrome.8 Cases were checked against the Regional Perinatal Mortality Survey to confirm the survival to 1 year.9 The Office for National Statistics provided maternal age data for our local population.
We included all cases where pregnancy reached at least 24 weeks and all terminations for fetal abnormality. Spontaneous losses before 24 weeks were noted separately but were not included in the figures for analysis because only a small and variable proportion of such losses are subjected to chromosomal analysis.6
Statistical analysis
Data are presented with means, medians, ranges and 95% confidence intervals. Categorical variables are expressed as absolute numbers (percentages) and comparisons between groups were made with Pearson's χ2-test. Poisson regression analysis was performed to determine whether trends in prevalence were significant.
Ethical approval
NorCAS is one of the seven linked surveys of fetal and infant health housed at the Regional Maternity Survey Office (RMSO) in Newcastle upon Tyne. The Patient Information Advisory Group (PIAG) has granted exemption from the requirement for consent for inclusion on the NorCAS register under section 60 of the Health and Social Care Act (2001). The RMSO has ethics approval (04/MRE04/25) to undertake studies involving the use of the data.
Results
Denominator population
In 1985–2004, there were 690 215 live births in the study population. Over 20 years, the birth rate fell by around 20%. (Figure 1)
Screening
Maternal serum screening for predicting the risk of Down syndrome was introduced as a pilot project in 1988 and more widely in 1991. The uptake in the Northern Region rose from 0% in 1987 to 40% of pregnancies by 1994 but has remained around 35–40% since then. (Figure 2) Recently other strategies such as nuchal translucency screening have been introduced. We have no data on the uptake of nuchal translucency screening.
There was a 39% increase in the number of mothers undergoing amniocentesis and a 94% increase in chorionic villus biopsy (Figure 2). The combined rate of amniocentesis and chorionic villus biopsy increased from <20 per 1000 total births in 1986–1988 to 35–40 per 1000 total births in 1997–2004. (Total births=live births+stillbirths.)
Down syndrome
In the 20 years of our study, Down syndrome affected 1188 pregnancies which either reached 24 weeks gestation or were terminated for fetal abnormality. The prevalence increased from 1.3 to 2.5 per 1000 total births. (Figure 3) Poisson regression analysis showed that the temporal trend in birth prevalence was highly significant (P<0.0001). Chromosomal analysis showed trisomy 21 in 1114, mosaicism in 30, translocation in 41, trisomy 21 plus XYY karyotype in two and trisomy 21 plus XXY karyotype in one. Down syndrome was also noted in 61 first trimester losses (not included in further analysis).
An antenatal diagnosis was made in 477/1188 cases (40%, 95% CI 37–43%) and was followed by termination of pregnancy in 389 (81%), stillbirth in 17 (4%) and live birth in 71 (15%). The decision to terminate the pregnancy after antenatal diagnosis varied with maternal age. The termination rate was 50/85 (59%, 95% CI 48–69%) for mothers aged less than 30 years, and 339/393 (86%, 95% CI 83–89%) aged 30 and above (χ2=34.7, P<0.0001).
Antenatal diagnosis was made in 17/51 stillbirths (33%) and 71/748 live births (9%). Antenatal diagnosis was made at a median gestation of 17 weeks (mean: 16, range: 10–24) in pregnancies ending in termination and at a median gestation of 20 weeks (mean: 23, range: 11–38) in pregnancies resulting in live birth.
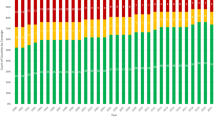
There were 748 live births (63%, 95%CI 60–66%) with Down syndrome (mean 1.08 per 1000 live births over 20 years). The live birth prevalence of Down syndrome declined from 1.16 in 1985–1989 to 0.94 per 1000 live births in 1990–1994 and increased from 1.03 in 1995–1999 to 1.21 per 1000 live births in 2000–2004 (P=0.01) with no overall change over 20 years (Figure 4). Cardiovascular malformations were found in 301 of 748 live births (40%).
Overall 1-year survival was 90% (87% in 1985–1994 and 94% in 1995–2004: P=0.003; Figure 4). Survival in infants with Down syndrome with normal hearts increased in the same two 10-year periods from 93 to 97% (P<0.05) and in those with cardiovascular malformations from 78 to 90% (P<0.0005).
Maternal age
The proportion of mothers of all live-born infants in the population who were 35 years or above at delivery rose from 6 to 15% over 20 years (Figure 1). The proportion of mothers who were 35 years or above in Down syndrome pregnancies was 30% in 1985–1989, 33% in 1990–1994, 44% in 1995–1999 and 52% in 2000–2004 (χ2= 37.91, P<0.0001).
Discussion
This study has shown complex changes over time in births with Down syndrome within a population. The total number of Down syndrome pregnancies is increasing, probably due mainly to the increasing proportion of older mothers. Live births with Down syndrome declined in the first half of our study, probably as a result of the introduction of serum screening and termination of pregnancy. In the second half of our study, live births with Down syndrome increased and infant mortality decreased, so there was an increase in long-term survivors. It seems likely that the influence of increasing maternal age outweighed the effect of antenatal diagnosis and termination of pregnancy in the second 10 years.
Our study represents a population with high ascertainment of Down syndrome pregnancies in comparison with other registries.5 Local data have been shown to have better ascertainment than the England and Wales national database.10
Another strength of this study is that we have data on the local uptake of maternal serum screening and amniocentesis. To our knowledge, Gidiri et al5 provide the only other UK data on the uptake of maternal serum screening, and they found a large reduction in the uptake over a similar time period. We did not find such a trend, but rather a plateau in the uptake, to a similar level of around 40%. This suggests that over the last 20 years, there have been wide regional variations in the uptake of maternal serum screening as well as trends over time. One factor relevant to the uptake but for which we do not have sufficient information to analyse is the nature of pretest counselling, and there have been recent efforts to standardise this as part of the National Health Service fetal anomaly screening programme (http://nscfa.web.its.manchester.ac.uk).
A further strength of our study is the inclusion of recent data on survival to one year. Rasmussen et al11 reported survival to 1 year in Atlanta between 1979 and 1998 to be 92.9% – comparable to our results. They had similar results with respect to the effect of cardiovascular malformations on survival to 1 year but do not provide data in trends in these factors over time.
How do our findings relate to those from other studies? Our study was based on the population of Northern England which is 97% white European. The proportion of mothers aged 35 or more years is about 2% lower than in the rest of England and Wales,12 so elsewhere the influence of maternal age may be greater than we have observed.
During most of the period under study, the availability of maternal serum screening was variable due to local variation in funding decisions. In 2001, the UK National Screening Committee was given responsibility to ensure a national programme was implemented in England by 2005. (www.screening.nhs.uk) Standardisation was therefore achieved in the Northern region of England after completion of this audit. The uptake of maternal serum screening in our region remains lower than in some others. In East Gloucestershire uptake, declined from 66 to 47% during the 1990s 13 but a survey in England in 2001 reported a wide variation.14
Previous studies have also demonstrated the effect on birth prevalence of Down syndrome of increasing maternal age. The proportion of mothers older than 34 years in Paris increased from 11% in 1981 to 26% in 2000.15 This rise led to an increase in Down syndrome pregnancies by 5% a year but increasing antenatal detection and termination of pregnancy resulted in an overall decrease in live births with Down syndrome of 3% a year. The rate of amniocentesis in Paris was greater than that in the rest of France or in the United States or United Kingdom, so the results of that study may not be directly comparable with other populations.
In the United States, the proportion of mothers aged 35 years or above increased from 5% in the 1970s to 14% in 2002. In a modelling exercise, Resta16 calculated that the proportion of Down syndrome live births to mothers 35 years or above would have increased from 25% in 1980 to 50% in 2002. Egan et al17 analysed birth certificate data from the United States. The estimated prevalence of Down syndrome pregnancies rose from 1.3–2.0 per 1000 live births in 1989–2001, but the estimated prevalence at live birth remained fairly constant over the same time (declining slightly from 0.98–0.91 per 1000 live births). These results are similar to our own for the same time period.
Dolk et al7 reported trends in the prevalence of Down syndrome in 14 European countries and documented a rise in the proportion of mothers who were 35 years or above from 8% in 1980–1984 to 14% in 1995–1999. There was wide variation in the rate of termination in the face of an antenatal diagnosis from 0–77%. Despite an increase in pregnancies with Down syndrome, the increase in terminations of pregnancy over time led to a stable or slightly reduced prevalence at live birth of Down syndrome. We have shown that the termination rate after prenatal diagnosis was lower in mothers younger than 30 years. Prenatal diagnosis in the younger mothers was more likely to have been based on ultrasound diagnosis of malformations with later karyotyping and therefore diagnosis of Down syndrome may have been made at a later gestation than for older mothers having an invasive test performed early.
Our report covers a later time frame than these published reports. We have perhaps a lower detection rate and a continuing rise in the proportion of older mothers. It may be that other populations will in time also show a net increase in live births with Down syndrome if maternal age continues to rise. A recent study from The Netherlands reports a live birth prevalence of Down syndrome in 2003 of 1.6 per 1000.18 Another recent report, on births in 1986–2004 in Australia, showed a slower rate of increase in total Down syndrome births than our series (with a slower increase in older mothers) and a fall in affected live births, so patterns vary in different populations.19 The increased use of nuchal translucency screening may contribute to a future decline in live births with Down syndrome, as has been documented recently in Denmark.20 The recent suggestions for improvement in antenatal counselling may also influence the uptake rates for screening.
Survival of infants with Down syndrome continues to improve.18, 21 In our recent experience, infant survival after live birth is now close to 100%. This is partly due to better surgical management of gastrointestinal and cardiovascular malformations. Long-term survival is also improving, and the large majority of people with Down syndrome are now expected to live well into adult life.22 Of those born 40 years ago, 75% now live to 40 and it is likely that the long-term survival of those born now will be much higher. Our data show an increasing number of infant survivors with Down syndrome over the last 10 years. Whatever future developments may occur in screening and whatever the resultant effect on live birth, there is currently a growing cohort of survivors with Down syndrome. In the long term, they have their own specific health problems, which are not well met by general resources for health care.23 In particular, adults with Down syndrome have an increased risk of endocrine, haematological, respiratory, neurological, psychiatric and social problems, which may require extra screening as well as extra health care resources. The current increasing number of children with Down syndrome will require adequate provision of health care throughout childhood and adult life.
References
Bower C, Leonard H, Petterson B : Intellectual disability in Western Australia. J Paediatr Child Health 2000; 36: 213–215.
Wyllie J, Madar R, Wright M, Burn J, Wren C : Strategies for antenatal detection of Down's syndrome. Arch Dis Child 1997; 76: F26–F30.
Leonard H, Wen X : The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev 2002; 8: 117–134.
Howe DT, Gornall R, Wellesley D, Boyle T, Barber J : Six year survey of screening for Down's syndrome by maternal age and mid-trimester ultrasound scans. BMJ 2000; 320: 606–610.
Gidiri M, McFarlane J, Holding S, Lindow S : Maternal serum screening for Down syndrome: are women's perceptions changing? BJOG 2007; 114: 458–461.
Richmond S, Atkins J : A population-based study of the prenatal diagnosis of congenital malformation over 16 years. BJOG 2005; 112: 1349–1357.
Dolk H, Loane M, Garne E et al: Trends and geographical inequalities in the prevalence of Down syndrome in Europe, 1980–1999. Rev Epidemiol Sante Publique 2005; 53: 2S87–2S95.
Wren C, Richmond S, Donaldson L : Presentation of congenital heart disease in infancy – implications for routine examination. Arch Dis Child 1999; 80: F49–F53.
Bell R, Glinianaia SV, Rankin J, Wright C, Pearce MS, Parker L : Changing patterns of perinatal death, 1982–2000; a retrospective cohort study. Arch Dis Child 2004; 89: F531–F536.
Boyd PA, Armstrong B, Dolk H et al: Congenital anomaly surveillance in England – ascertainment deficiencies in the national system. BMJ 2005; 330: 27–31.
Rasmussen S, Wong L, Correa A, Gambrell D, Friedman J : Survival in infants with Down syndrome, Metropolitan Atlanta, 1979–1998. J Pediatr 2006; 148: 806–812.
Office for National Statistics: Live births: 1938–2004. accessed at http://www.statistics.gov.uk/STATBASE/xsdataset.asp?vlnk=4274.
Dixon J, Pillai M, Mahendran D, Brooks M : An assessment of the Down syndrome antenatal screening policies of East and West Gloucestershire between 1993 and 1999. J Obstet Gynaecol 2004; 24: 760–764.
Ward P, Muir Gray JA : Antenatal screening service for Down's syndrome in England: 2001. National Screening Committee. available at http://www.library.nhs.uk/screening/viewResource.aspx?resID=59830.
Khoshnood B, De Vigan C, Vodovar V, Goujard J, Goffinet F : A population-based evaluation of the impact of antenatal screening for Down's syndrome in France, 1981–2000. BJOG 2004; 111: 485–490.
Resta RG : Changing demographics of advanced maternal age (AMA) and the impact on the predicted incidence of Down syndrome in the United States: implications for prenatal screening and genetic counseling. Am J Med Genet 2005; 133A: 31–36.
Egan JFX, Benn PA, Zelop CM, Bolnick A, Gianferrari E, Borgida AF : Down syndrome births in the United States from 1989 to 2001. Am J Obstet Gynecol 2004; 191: 1044–1048.
Weijerman ME, van Furth AM, Noordegraaf AV, van Wouwe JP, Broers CJM, Gemke RJBJ : Prevalence, neonatal characteristics, and first-year mortality of Down syndrome: a national study. J Pediatr 2008; 152: 15–19.
Collins VR, Muggli EE, Riley M, Palma S, Halliday JL : Is Down syndrome a disappearing birth defect? J Pediatr 2008; 152: 20–24.
Mayor S : Denmark halves Down's births by non-invasive screening in early pregnancy. BMJ 2007; 334: 1291.
Merrick J : Down syndrome in Israel. Downs Syndr Res Pract 2001; 6: 128–130.
Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD, Bittles AH : The changing survival profile of people with Down's syndrome: implications for genetic counselling. Clin Genet 2002; 62: 390–393.
Smith DS : Health care management of adults with Down's syndrome. Am Fam Physician 2001; 64: 1031–1038.
Acknowledgements
We are indebted to Mary Bythell at the Northern Congenital Abnormality Survey and the Perinatal Mortality Survey for providing data, and to Professor John Matthews for statistical advice. We are grateful to all link clinicians in the Northern region for their continued collaboration and support of the NorCAS. NorCAS is funded by the Department of Health Policy Research Programme (Disease Register). All authors participated in the design, data analysis and writing of the study and have seen and approved the final version. Claire Irving and Anna Basu collected most of the data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
All authors declare that they have no conflict of interest and have nothing to declare. No funding was obtained for this study.
Rights and permissions
About this article
Cite this article
Irving, C., Basu, A., Richmond, S. et al. Twenty-year trends in prevalence and survival of Down syndrome. Eur J Hum Genet 16, 1336–1340 (2008). https://doi.org/10.1038/ejhg.2008.122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2008.122
Keywords
This article is cited by
-
The Role of Oxidative Stress in Trisomy 21 Phenotype
Cellular and Molecular Neurobiology (2023)
-
“Tell Me About Your Child, The Relationship with Your Child and Your Parental Experiences”: A Qualitative Study of Spontaneous Speech Samples Among Parents Raising a Child with and without Autism Spectrum Disorder, Cerebral Palsy or Down Syndrome
Journal of Developmental and Physical Disabilities (2022)
-
Infection risk among adults with down syndrome: a two group series of 101 patients in a tertiary center
Orphanet Journal of Rare Diseases (2019)
-
Ten-year trend in prevalence and outcome of Down syndrome with congenital heart disease in a middle-income country
European Journal of Pediatrics (2019)
-
Mouse models of Down syndrome: gene content and consequences
Mammalian Genome (2016)