Abstract
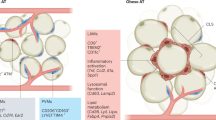
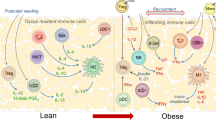
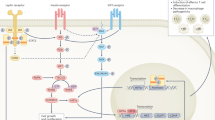
Diabetes mellitus is one of the most common chronic metabolic disorders worldwide, and its incidence in Asian countries is alarmingly high. Type 2 diabetes (T2DM) is closely associated with obesity, and the staggering rise in obesity is one of the primary factors related to the increased frequency of T2DM. Low-grade chronic inflammation is also accepted as an integral metabolic adaption in obesity and T2DM, and is believed to be a major player in the onset of insulin resistance. However, the exact mechanism(s) that cause a persistent chronic low-grade infiltration of leukocytes into insulin-target tissues such as adipose, skeletal muscle and liver are not entirely known. Recent developments in the understanding of leukocyte metabolism have revealed that the inflammatory polarization of immune cells, and consequently their immunological function, are strongly connected to their metabolic profile. Therefore, it is hypothesized that dysfunctional immune cell metabolism is a central cellular mechanism that prevents the resolution of inflammation in chronic metabolic conditions such as that observed in obesity and T2DM. The purpose of this review is to explore the metabolic demands of different immune cell types, and identify the molecular switches that control immune cell metabolism and ultimately function. Understanding of these concepts may allow the development of interventions that can correct immune function and may possibly decrease chronic low-grade inflammation in humans suffering from obesity and T2DM. We also review the latest clinical techniques used to measure metabolic flux in primary leukocytes isolated from obese and T2DM patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jayawardena R, Ranasinghe P, Byrne NM, Soares MJ, Katulanda P, Hills AP . Prevalence and trends of the diabetes epidemic in South Asia: a systematic review and meta-analysis. BMC Public Health 2012; 12: 380.
Shaw JE, Sicree RA, Zimmet PZ . Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14.
Bongaarts J . Human population growth and the demographic transition. Philos Trans R Soc Lond B Biol Sci 2009; 364: 2985–2990.
Hu FB . Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011; 34: 1249–1257.
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016; 387: 1513–1530.
Baker RG, Hayden MS, Ghosh S . NF-κB, inflammation, and metabolic disease. Cell Metab 2016; 13: 11–22.
Calton EK, James AP, Pannu PK, Soares MJ . Certain dietary patterns are beneficial for the metabolic syndrome: reviewing the evidence. Nutr Res 2014; 34: 559–568.
Pingali P . Westernization of Asian diets and the transformation of food systems: implications for research and policy. Food Policy 2007; 32: 281–298.
Thoudam T, Jeon J-H, Ha C-M, Lee I-K . Role of mitochondria-associated endoplasmic reticulum membrane in inflammation-mediated metabolic diseases. Mediators Inflamm 2016; 2016: 18.
Trayhurn P . Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 2013; 93: 1–21.
Johnson AR, Milner JJ, Makowski L . The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev 2012; 249: 218–238.
Odegaard JI, Chawla A . Alternative macrophage activation and metabolism. Annu Rev Pathol 2011; 6: 275–297.
Enos RT, Davis JM, Velazquez KT, McClellan JL, Day SD, Carnevale KA et al. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res 2013; 54: 152–163.
Teng K-T, Chang C-Y, Chang LF, Nesaretnam K . Modulation of obesity-induced inflammation by dietary fats: mechanisms and clinical evidence. Nutr J 2014; 13: 12.
McArdle M, Finucane O, Connaughton R, McMorrow A, Roche H . Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol 2013; 4: 52.
Calton EK, Keane K, Soares MJ . The potential regulatory role of vitamin D in the bioenergetics of inflammation. Curr Opin Clin Nutr Metab Care 2015; 18: 367–373.
Liu TF, Brown CM, El Gazzar M, McPhail L, Millet P, Rao A et al. Fueling the flame: bioenergy couples metabolism and inflammation. J Leukoc Biol 2012; 92: 499–507.
Buck MD, O'Sullivan D, Pearce EL . T cell metabolism drives immunity. J Exp Med 2015; 212: 1345–1360.
Norata GD, Caligiuri G, Chavakis T, Matarese G, Netea MG, Nicoletti A et al. The cellular and molecular basis of translational immunometabolism. Immunity 2015; 43: 421–434.
O'Neill LA, Pearce EJ . Immunometabolism governs dendritic cell and macrophage function. J Exp Med 2016; 213: 15–23.
O'Neill LA, Kishton RJ, Rathmell J . A guide to immunometabolism for immunologists. Nat Rev Immunol 2016; 16: 553–565.
Freitag J, Berod L, Kamradt T, Sparwasser T . Immunometabolism and autoimmunity. Immunol Cell Biol 2016; 94: 925–934.
Chang C-H, Pearce EL . Emerging concepts in immunotherapy – T cell metabolism as a therapeutic target. Nat Immunol 2016; 17: 364–368.
Chacko BK, Kramer PA, Ravi S, Johnson MS, Hardy RW, Ballinger SW et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest 2013; 93: 690–700.
O'Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 2014; 41: 75–88.
Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM . A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol 2014; 2: 206–210.
Pearce EJ, Everts B . Dendritic cell metabolism. Nat Rev Immunol 2015; 15: 18–29.
Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010; 115: 4742–4749.
Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat Immunol 2014; 15: 323–332.
Maroof A, English NR, Bedford PA, Gabrilovich DI, Knight SC . Developing dendritic cells become 'lacy' cells packed with fat and glycogen. Immunology 2005; 115: 473–483.
Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, Yarasheski KE et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood 2012; 120: 1422–1431.
Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol 2010; 185: 605–614.
Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction, and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab 2015; 21: 65–80.
Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 2011; 145: 732–744.
Infantino V, Convertini P, Cucci L, Panaro MA, Di Noia MA, Calvello R et al. The mitochondrial citrate carrier: a new player in inflammation. Biochem J 2011; 438: 433–436.
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013; 496: 238–242.
Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR et al. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab 2006; 4: 13–24.
Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015; 42: 419–430.
Canto C, Auwerx J . PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 2009; 20: 98–105.
Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PIH . Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J 2016; 473: 4527–4550.
Nakaya M, Xiao Y, Zhou X, Chang JH, Chang M, Cheng X et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 2014; 40: 692–705.
Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab 2012; 15: 110–121.
Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol 2013; 14: 1064–1072.
Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol 2015; 16: 188–196.
Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 2011; 208: 1367–1376.
Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med 2014; 20: 1327–1333.
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 2011; 186: 3299–3303.
Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest 2015; 125: 194–207.
van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 2012; 36: 68–78.
Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009; 30: 832–844.
Procaccini C, De Rosa V, Galgani M, Carbone F, Cassano S, Greco D et al. Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J Immunol 2012; 189: 2941–2953.
Soliman GA . The role of mechanistic target of rapamycin (mTOR) complexes signaling in the immune responses. Nutrients 2013; 5: 2231–2257.
Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A et al. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and functionin vivo. Blood 2003; 101: 4457–4463.
Wise DR, Thompson CB . Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 2010; 35: 427–433.
Newsholme P . Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr 2001; 131 (9 Suppl), 2515S–2522S. discussion 23S-4S.
Palazon A, Goldrath AW, Nizet V, Johnson RS . HIF transcription factors, inflammation, and immunity. Immunity 2014; 41: 518–528.
Marin-Hernandez A, Gallardo-Perez JC, Ralph SJ, Rodriguez-Enriquez S, Moreno-Sanchez R . HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem 2009; 9: 1084–1101.
Kominsky DJ, Campbell EL, Colgan SP . Metabolic shifts in immunity and inflammation. J Immunol 2010; 184: 4062–4068.
Philip B, Ito K, Moreno-Sanchez R, Ralph SJ . HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis 2013; 34: 1699–1707.
Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E . Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem 2002; 277: 27975–27981.
O'Neill LA, Hardie DG . Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013; 493: 346–355.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P . Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005; 434: 113–118.
Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 2010; 327: 1004–1007.
Liu TF, Yoza BK, El Gazzar M, Vachharajani VT, McCall CE . NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J Biol Chem 2011; 286: 9856–9864.
McCall CE, Yoza B, Liu T, El Gazzar M . Gene-specific epigenetic regulation in serious infections with systemic inflammation. J Innate Immun 2010; 2: 395–405.
Frede S, Stockmann C, Winning S, Freitag P, Fandrey J . Hypoxia-inducible factor (HIF) 1alpha accumulation and HIF target gene expression are impaired after induction of endotoxin tolerance. J Immunol 2009; 182: 6470–6476.
Svajger U, Obermajer N, Jeras M . Dendritic cells treated with resveratrol during differentiation from monocytes gain substantial tolerogenic properties upon activation. Immunology 2010; 129: 525–535.
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006; 127: 1109–1122.
Pecht T, Gutman-Tirosh A, Bashan N, Rudich A . Peripheral blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes Rev 2014; 15: 322–337.
Hotamisligil GS, Shargill NS, Spiegelman BM . Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259: 87–91.
Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM . Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409–2415.
Schmidt FM, Weschenfelder J, Sander C, Minkwitz J, Thormann J, Chittka T et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One 2015; 10: e0121971.
Marques-Vidal P, Bastardot F, von Kanel R, Paccaud F, Preisig M, Waeber G et al. Association between circulating cytokine levels, diabetes and insulin resistance in a population-based sample (CoLaus study). Clin Endocrinol 2013; 78: 232–241.
Panee J . Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012; 60: 1–12.
Calton EK, Keane KN, Soares MJ, Rowlands J, Newsholme P . Prevailing vitamin D status influences mitochondrial and glycolytic bioenergetics in peripheral blood mononuclear cells obtained from adults. Redox Biol 2016; 10: 243–250.
Donath MY . Targeting inflammation in the treatment of type 2 diabetes. Diabetes Obes Metab 2013; 15 (Suppl 3), 193–196.
Keane KN, Cruzat VF, Carlessi R, de Bittencourt PIH, Newsholme P . Molecular events linking oxidative stress and inflammation to insulin resistance and beta-cell dysfunction. Oxid Med Cell Longev 2015; 2015: 15.
Hotamisligil GS . Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010; 140: 900–917.
Back SH, Kaufman RJ . Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem 2012; 81: 767–793.
Cameron NE . Role of endoplasmic reticulum stress in diabetic neuropathy. Diabetes 2013; 62: 696–697.
Jeschke MG, Boehning D . Endoplasmic reticulum stress and insulin resistance post-trauma: similarities to type 2 diabetes. J Cell Mol Med 2012; 16: 437–444.
Hartman M-L, Shirihai OS, Holbrook M, Xu G, Kocherla M, Shah A et al. Relation of mitochondrial oxygen consumption in peripheral blood mononuclear cells to vascular function in type 2 diabetes mellitus. Vasc Med 2014; 19: 67–74.
Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM . A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol 2014; 2: 206–210.
O'Grada CM, Morine MJ, Morris C, Ryan M, Dillon ET, Walsh M et al. PBMCs reflect the immune component of the WAT transcriptome–implications as biomarkers of metabolic health in the postprandial state. Mol Nutr Food Res 2014; 58: 808–820.
Tyrrell DJ, Bharadwaj MS, Jorgensen MJ, Register TC, Molina AJ . Blood cell respirometry is associated with skeletal and cardiac muscle bioenergetics: implications for a minimally invasive biomarker of mitochondrial health. Redox Biol 2016; 10: 65–77.
Maynard S, Hejl AM, Dinh TS, Keijzers G, Hansen AM, Desler C et al. Defective mitochondrial respiration, altered dNTP pools and reduced AP endonuclease 1 activity in peripheral blood mononuclear cells of Alzheimer's disease patients. Aging 2015; 7: 793–815.
Tyrrell DJ, Bharadwaj MS, Van Horn CG, Marsh AP, Nicklas BJ, Molina AJ . Blood-cell bioenergetics are associated with physical function and inflammation in overweight/obese older adults. Exp Gerontol 2015; 70: 84–91.
Biniecka M, Canavan M, McGarry T, Gao W, McCormick J, Cregan S et al. Dysregulated bioenergetics: a key regulator of joint inflammation. Ann Rheum Dis 2016; 75: 2192–2200.
Keane KN, Calton EK, Cruzat VF, Soares MJ, Newsholme P . The impact of cryopreservation on human peripheral blood leucocyte bioenergetics. Clin Sci 2015; 128: 723–733.
Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ et al. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res 2010; 156: 15–25.
Czajka A, Ajaz S, Gnudi L, Parsade CK, Jones P, Reid F et al. Altered mitochondrial function, mitochondrial DNA and reduced metabolic flexibility in patients with diabetic nephropathy. EBioMedicine 2015; 2: 499–512.
Chacko BK, Kramer PA, Ravi S, Benavides GA, Mitchell T, Dranka BP et al. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin Sci 2014; 127: 367–373.
Krause M, Keane K, Rodrigues-Krause J, Crognale D, Egan B, De Vito G et al. Elevated levels of extracellular heat-shock protein 72 (eHSP72) are positively correlated with insulin resistance in vivo and cause pancreatic beta-cell dysfunction and death in vitro. Clin Sci 2014; 126: 739–752.
Acknowledgements
We thank the School of Biomedical Sciences and School of Public Health in the Faculty of Health Sciences at Curtin University, along with Telethon Kids Institute, University of Western Australia, Perth, Australia for research support. Curtin Health Innovation Research Institute (CHIRI) is acknowledged with thanks for access to excellent research facilities. EKC is the recipient of an Australian postgraduate scholarship award (APA).
Author contributions
The present work was designed by KNK. Initial manuscript preparation and draft was undertaken by KNK, EKC and PHH, and subsequently revised by RC and PN. Figures were designed by KNK, EKC and RC, and prepared by KNK and EKC. All authors approved the final version of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Keane, K., Calton, E., Carlessi, R. et al. The bioenergetics of inflammation: insights into obesity and type 2 diabetes. Eur J Clin Nutr 71, 904–912 (2017). https://doi.org/10.1038/ejcn.2017.45
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2017.45
This article is cited by
-
Progress and application of adipose-derived stem cells in the treatment of diabetes and its complications
Stem Cell Research & Therapy (2024)
-
Glucose-lowering effects of orally administered superoxide dismutase in type 2 diabetic model rats
npj Science of Food (2022)
-
Comparative Analysis of the Therapeutic Effects of Amniotic Membrane and Umbilical Cord Derived Mesenchymal Stem Cells for the Treatment of Type 2 Diabetes
Stem Cell Reviews and Reports (2022)
-
Mesenchymal Stem Cell-Based Therapy for Diabetes Mellitus: Enhancement Strategies and Future Perspectives
Stem Cell Reviews and Reports (2021)
-
The oral microbiome profile and biomarker in Chinese type 2 diabetes mellitus patients
Endocrine (2020)