Abstract
Background/Objectives:
There has been recent interest in barley as a therapeutic food owing to its high content of beta-glucan (β-glucan), a viscous soluble fiber recognized for its cholesterol-lowering properties. The objective of this study was to conduct a systematic review and meta-analysis of randomized controlled trials (RCTs) investigating the cholesterol-lowering potential of barley β-glucan on low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C) and apolipoprotein B (apoB) for cardiovascular disease (CVD) risk reduction.
Methods:
MEDLINE, Embase, CINAHL and the Cochrane CENTRAL were searched. We included RCTs of ⩾3-week duration assessing the effect of diets enriched with barley β-glucan compared with controlled diets on LDL-C, non-HDL-C or apoB. Two independent reviewers extracted relevant data and assessed study quality and risk of bias. Data were pooled using the generic inverse-variance method with random effects models and expressed as mean differences (MDs) with 95% confidence intervals (CIs). Heterogeneity was assessed by the Cochran Q-statistic and quantified by the I2 statistic.
Results:
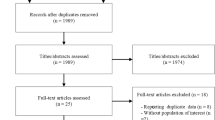
Fourteen trials (N=615) were included in the final analysis. A median dose of 6.5 and 6.9 g/day of barley β-glucan for a median duration of 4 weeks significantly reduced LDL-C (MD=−0.25 mmol/l (95% CI: −0.30, −0.20)) and non-HDL-C (MD=−0.31 mmol/l (95% CI: −0.39, −0.23)), respectively, with no significant changes to apoB levels, compared with control diets. There was evidence of considerable unexplained heterogeneity in the analysis of non-HDL-C (I2=98%).
Conclusions:
Pooled analyses show that barley β-glucan has a lowering effect on LDL-C and non-HDL-C. Inclusion of barley-containing foods may be a strategy for achieving targets in CVD risk reduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
02 November 2016
This article has been corrected since Advance Online Publication and a corrigendum is also printed in this issue
References
Brown L, Rosner B, Willett WW, Sacks FM . Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999; 69: 30–42.
Ripsin CM, Keenan JM, Jacobs Jr DR, Elmer PJ, Welch RR, Van Horn L et al. Oat products and lipid lowering. A meta-analysis. JAMA 1992; 267: 3317–3325.
de Groot A, Luyken R, Pikaar NA . Cholesterol-lowering effect of rolled oats. Lancet 1963; 2: 303–304.
Newman RK, Lewis SE, Newman CW, Boik RJ, Ramage RT . Hypocholesterolemic effect of barley foods on healthy men. Nutrition Reports International 1989; 39: 749–760.
Ezatagha A, The effect of Barley Beta-Glucan concentrate on LDL-Cholesterol and other risk factors for cardiovascular disease (thesis): University of Toronto; 2007.
Behall KM, Scholfield DJ, Hallfrisch J . Diets containing barley significantly reduce lipids in mildly hypercholesterolemic men and women. Am J Clin Nutr 2004; 80: 1185–1193.
Behall KM, Scholfield DJ, Hallfrisch J . Lipids significantly reduced by diets containing barley in moderately hypercholesterolemic men. J Am Coll Nutr 2004; 23: 55–62.
Keenan JM, Goulson M, Shamliyan T, Knutson N, Kolberg L, Curry L . The effects of concentrated barley beta-glucan on blood lipids in a population of hypercholesterolaemic men and women. Br J Nutr 2007; 97: 1162–1168.
Li J, Kaneko T, Qin LQ, Wang J, Wang Y . Effects of barley intake on glucose tolerance, lipid metabolism, and bowel function in women. Nutrition 2003; 19: 926–929.
Lupton JR, Robinson MC, Morin JL . Cholesterol-lowering effect of barley bran flour and oil. J Am Diet Assoc 1994; 94: 65–70.
McIntosh GH, Whyte J, McArthur R, Nestel PJ . Barley and wheat foods: influence on plasma cholesterol concentrations in hypercholesterolemic men. Am J Clin Nutr 1991; 53: 1205–1209.
Rondanelli M, Opizzi A, Monteferrario F, Klersy C, Cazzola R, Cestaro B . Beta-glucan- or rice bran-enriched foods: a comparative crossover clinical trial on lipidic pattern in mildly hypercholesterolemic men. Eur J Clin Nutr 2011; 65: 864–871.
Shimizu C, Kihara M, Aoe S, Araki S, Ito K, Hayashi K et al. Effect of high beta-glucan barley on serum cholesterol concentrations and visceral fat area in Japanese men—a randomized, double-blinded, placebo-controlled trial. Plant Foods Hum Nutr 2008; 63: 21–25.
Sundberg B . Cholesterol lowering effects of a barley fibre flake product. Agro Food Industry Hi-Tech 2008; 19: 14–17.
Biorklund M, van Rees A, Mensink RP, Onning G . Changes in serum lipids and postprandial glucose and insulin concentrations after consumption of beverages with beta-glucans from oats or barley: a randomised dose-controlled trial. Eur J Clin Nutr 2005; 59: 1272–1281.
Keogh GF, Cooper GJ, Mulvey TB, McArdle BH, Coles GD, Monro JA et al. Randomized controlled crossover study of the effect of a highly beta-glucan-enriched barley on cardiovascular disease risk factors in mildly hypercholesterolemic men. Am J Clin Nutr 2003; 78: 711–718.
Ibrugger S, Kristensen M, Poulsen MW, Mikkelsen MS, Ejsing J, Jespersen BM et al. Extracted oat and barley beta-glucans do not affect cholesterol metabolism in young healthy adults. J Nutr 2013; 143: 1579–1585.
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2011; 9: 2207.
Food Directorate, Health Products and Food Branch, Health Canada. Barley Products and Blood Cholesterol Lowering. Bureau of Nutritional Sciences: Health Canada, Ottawa, ON, Canada, 2012.
National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421.
Anderson TJ, Gregoire J, Hegele RA, Couture P, Mancini GB, McPherson R et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2012; 29: 151–167.
Cochrane Handbook for Systematic Reviews of Interventions 2011. Available from www.cochrane-handbook.org.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097.
Food and Drug Administration Guidance for Industry: Evidence-based review system for the scientific evaluation of health claims - final. 2009.
Greer N, Mosser G, Logan G, Halaas GW . A practical approach to evidence grading. Jt Comm J Qual Improv 2000; 26: 700–712.
Kris-Etherton PM, Dietschy J . Design criteria for studies examining individual fatty acid effects on cardiovascular disease risk factors: human and animal studies. Am J Clin Nutr 1997; 65 (5 Suppl), 1590s–1596s.
Limberger-Bayer VM, de Francisco A, Chan A, Oro T, Ogliari PJ, Barreto PL . Barley b-glucans extraction and partial characterization. Food Chem 2014; 154: 84–89.
Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U . Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001; 286: 944–953.
Ha V . Effects of Dietary Pulses on Lipid Risk Factors of Cardiovascular Disease and Oxidative Stress (MSc Thesis): University of Toronto; 2013.
Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A . Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002; 31: 140–149.
Talati R, Baker WL, Pabilonia MS, White CM, Coleman CI . The effects of barley-derived soluble fiber on serum lipids. Ann Fam Med 2009; 7: 157–163.
AbuMweis SS, Jew S, Ames NP . Beta-glucan from barley and its lipid-lowering capacity: a meta-analysis of randomized, controlled trials. Eur J Clin Nutr 2010; 64: 1472–1480.
Saenger A . Cardiovascular Risk Assessment Beyond LDL Cholesterol: Non-HDL Cholesterol, LDL Particle Number, and Apolipoprotein B. Mayo Clinic Cimmunique Articles 2011, Available from http://www.mayomedicallaboratories.com/articles/communique/2011/11.html.
Newman RKN, Barley CW . Genetics and Nutrient Composition. Barley for Food and Health: Science, Technology, and Products. John Wiley & Sons, Inc: Hoboken, NJ, USA, 2008. pp 56–94.
Theuwissen E, Mensink RP . Water-soluble dietary fibers and cardiovascular disease. Physiol Behav 2008; 94: 285–292.
Vuksan V, Jenkins AL, Rogovik AL, Fairgrieve CD, Jovanovski E, Leiter LA . Viscosity rather than quantity of dietary fibre predicts cholesterol-lowering effect in healthy individuals. Br J Nutr 2011; 106: 1349–1352.
Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005; 294: 2455–2464.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
HVTH, AZ, SBM, EJ and FAY declare no conflicts of interest related to this manuscript. JLS has received research support from the Canadian Institutes of health Research (CIHR), Canadian Diabetes Association, PSI Foundation, Calorie Control Council, American Society of Nutrition (ASN), The Coca-Cola Company (investigator initiated, unrestricted), Dr Pepper Snapple Group (investigator initiated, unrestricted), Pulse Canada, and The International Tree Nut Council Nutrition Research & Education Foundation, and the INC International Nut and Dried Fruit Council. He has received reimbursement of travel expenses, speaker fees and/or honoraria from the American Heart Association (AHA), American College of Physicians (ACP), American Society for Nutrition (ASN), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Canadian Diabetes Association (CDA), Canadian Nutrition Society (CNS), University of South Carolina, University of Alabama at Birmingham, Oldways Preservation Trust, Nutrition Foundation of Italy (NFI), Calorie Control Council, Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), International Life Sciences Institute (ILSI) North America, International Life Sciences Institute (ILSI) Brazil, Abbott Laboratories, Pulse Canada, Canadian Sugar Institute, Dr Pepper Snapple Group, The Coca-Cola Company, Corn Refiners Association, World Sugar Research Organization, Dairy Farmers of Canada, Società Italiana di Nutrizione Umana (SINU), III World Congress of Public Health Nutrition, C3 Collaborating for Health, White Wave Foods, Rippe Lifestyle and mdBriefcase. He has ad hoc consulting arrangements with Winston & Strawn LLP, Perkins Coie LLP and Tate & Lyle. He is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of both the Canadian Diabetes Association (CDA) European Association for the study of Diabetes (EASD), and Canadian Cardiovascular Society (CCS), as well as being on an American Society for Nutrition (ASN) writing panel for a scientific statement on sugars. He is a member of the International Carbohydrate Quality Consortium (ICQC) and Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD. He serves an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America. His wife is an employee of Unilever Canada. VV holds a research grant from CDA for study of dietary intervention including viscous soluble fiber and holds the Canadian (2,410,556) and American (7,326.404) patent on the medical use of viscous fiber blend for reducing blood glucose for treatment of diabetes, increasing insulin sensitivity, reduction in systolic blood pressure and blood lipids. ALJ is director of research and part owner of Glycemic Index Laboratories, a clinical research organization.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Rights and permissions
About this article
Cite this article
Ho, H., Sievenpiper, J., Zurbau, A. et al. A systematic review and meta-analysis of randomized controlled trials of the effect of barley β-glucan on LDL-C, non-HDL-C and apoB for cardiovascular disease risk reductioni-iv. Eur J Clin Nutr 70, 1239–1245 (2016). https://doi.org/10.1038/ejcn.2016.89
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2016.89
This article is cited by
-
Characterisation of barley landraces from Syria and Jordan for resistance to rhynchosporium and identification of diagnostic markers for Rrs1Rh4
Theoretical and Applied Genetics (2020)
-
Cholesterol-Lowering Nutraceuticals Affecting Vascular Function and Cardiovascular Disease Risk
Current Cardiology Reports (2018)