Abstract
Congenital hypogonadotropic hypogonadism (CHH) causes pubertal failure and infertility in both women and men due to partial or total secretory failure of the two pituitary gonadotropins lutropin (LH) and follitropin (FSH) during periods of physiological activation of the gonadotropic axis. Men and women with CHH frequently seek treatment for infertility after hypogonadism therapy. Some etiologies, such as autosomal dominant or X-linked Kallmann syndrome, raise the question of hereditary transmission, leading to increasing demands for genetic counseling and monitoring of medically assisted pregnancies. Diagnosis and treatment of newborn boys is, therefore, becoming an increasingly important issue. In male individuals with complete forms of CHH, the antenatal and neonatal gonadotropin deficit leads to formation of a micropenis and cryptorchidism, which could undermine future sexual and reproductive functions. Standard treatments, usually started after the age of puberty, often only partially correct the genital abnormalities and spermatogenesis. The aim of this Review is to examine the possible additional benefits of neonatal gonadotropin therapy in male patients with CHH. Encouraging results of neonatal therapy, together with a few reports of prepubertal treatment, support the use of this novel therapeutic strategy aimed at improving sexual and reproductive functions in adulthood.
Key Points
-
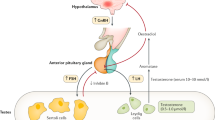
During fetal life, hypothalamic gonadotropin-releasing hormone (GnRH) and pituitary gonadotropins play a key part in the development and growth of the male external genitalia
-
Complete congenital hypogonadotropic hypogonadism (CHH) is associated with penile and testicular hypotrophy and, in many cases, with cryptorchidism
-
Conventional treatment of patients with CHH, with testosterone or gonadotropins, is inadequately effective in terms of adult sexuality and fertility when started after the age of puberty
-
Neonatal treatment corrects genital hypotrophy and improves testicular endocrine function, which might improve the response to treatments intended to induce postpubertal virilization and to restore fertility in men with CHH
-
Long-term studies are needed to assess the effect of this approach on sexuality and fertility before recommending its routine use
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Brioude, F. et al. Non-syndromic congenital hypogonadotropic hypogonadism: clinical presentation and genotype–phenotype relationships. Eur. J. Endocrinol. 162, 835–851 (2010).
Bianco, S. D. & Kaiser, U. B. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat. Rev. Endocrinol. 5, 569–576 (2009).
Mitchell, A. L., Dwyer, A., Pitteloud, N. & Quinton, R. Genetic basis and variable phenotypic expression of Kallmann syndrome: towards a unifying theory. Trends Endocrinol. Metab. 22, 249–258 (2011).
Dodé, C. & Hardelin, J. P. Kallmann syndrome. Eur. J. Hum. Genet. 17, 139–146 (2009).
Kim, H. G. et al. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am. J. Hum. Genet. 83, 511–519 (2008).
Jongmans, M. C. et al. CHD7 mutations in patients initially diagnosed with Kallmann syndrome—the clinical overlap with CHARGE syndrome. Clin. Genet. 75, 65–71 (2009).
Sarfati, J., Dodé, C. & Young, J. Kallmann syndrome caused by mutations in the PROK2 and PROKR2 genes: pathophysiology and genotype–phenotype correlations. Front. Horm. Res. 39, 121–132 (2010).
Shoham, Z., Smith, H., Yeko, T., O'Brien, F., Hemsey, G. & O'Dea, L. Recombinant LH (lutropin alfa) for the treatment of hypogonadotrophic women with profound LH deficiency: a randomized, double-blind, placebo-controlled, proof-of-efficacy study. Clin. Endocrinol. (Oxf.) 69, 471–478 (2008).
Scott, H. M., Mason, J. I. & Sharpe, R. M. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr. Rev. 30, 883–925 (2009).
O'Shaughnessy, P. J. & Fowler, P. A. Endocrinology of the mammalian fetal testis. Reproduction 141, 37–46 (2011).
Molsberry, R. L., Carr, B. R., Mendelson, C. R. & Simpson, E. R. Human chorionic gonadotropin binding to human fetal testes as a function of gestational age. J. Clin. Endocrinol. Metab. 55, 791–794 (1982).
Fowler, P. A., Bhattacharya, S., Gromoll, J., Monteiro, A. & O'Shaughnessy, P. J. Maternal smoking and developmental changes in luteinizing hormone (LH) and the LH receptor in the fetal testis. J. Clin. Endocrinol. Metab. 94, 4688–4695 (2009).
Latronico, A. C. & Arnhold, I. J. Inactivating mutations of LH and FSH receptors—from genotype to phenotype. Pediatr. Endocrinol. Rev. 4, 28–31 (2006).
Mulchahey, J. J., Di Blasio, A. M., Martin, M. C., Blumenfeld, Z. & Jaffe, R. B. Hormone production and peptide regulation of the human fetal pituitary gland. Endocr. Rev. 8, 406–425 (1987).
Kletzky, O. A., Rossman, F., Bertolli, S. I., Platt, L. D. & Mishell, D. R. Jr. Dynamics of human chorionic gonadotropin, prolactin, and growth hormone in serum and amniotic fluid throughout normal human pregnancy. Am. J. Obstet. Gynecol. 151, 878–884 (1985).
Hutson, J. M. & Donahoe, P. K. The hormonal control of testicular descent. Endocr. Rev. 7, 270–283 (1986).
Foresta, C., Zuccarello, D., Garolla, A. & Ferlin, A. Role of hormones, genes, and environment in human cryptorchidism. Endocr. Rev. 29, 560–580 (2008).
Bay, K., Main, K. M., Toppari, J. & Skakkebæk, N. E. Testicular descent: INSL3, testosterone, genes and the intrauterine milieu. Nat. Rev. Urol. 8, 187–196 (2011).
Plant, T. M. & Marshall, G. R. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr. Rev. 22, 764–786 (2001).
Huhtaniemi, I. T., Yamamoto, M., Ranta, T., Jalkanen, J. & Jaffe, R. B. Follicle-stimulating hormone receptors appear earlier in the primate fetal testis than in the ovary. J. Clin. Endocrinol. Metab. 65, 1210–1214 (1987).
O'Shaughnessy, P. et al. Developmental changes in human fetal testicular cell numbers and messenger ribonucleic acid levels during the second trimester. J. Clin. Endocrinol. Metab. 92, 4792–4801 (2007).
Boukari, K. et al. Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testis development. J. Clin. Endocrinol. Metab. 94, 1818–1825 (2009).
Debieve, F., Beerlandt, S., Hubinont, C. & Thomas, K. Gonadotropins, prolactin, inhibin A, inhibin B, and activin A in human fetal serum from midpregnancy and term pregnancy. J. Clin. Endocrinol. Metab. 85, 270–274 (2000).
Mesiano, S., Hart, C. S., Heyer, B. W., Kaplan, S. L. & Grumbach, M. M. Hormone ontogeny in the ovine fetus. XXVI. A sex difference in the effect of castration on the hypothalamic–pituitary gonadotropin unit in the ovine fetus. Endocrinology. 129, 3073–3079 (1991).
Brandenberger, A. W., Tee, M. K., Lee, J. Y., Chao, V. & Jaffe, R. B. Tissue distribution of estrogen receptors α (ER-α) and β (ER-β) mRNA in the midgestational human fetus. J. Clin. Endocrinol. Metab. 82, 3509–3512 (1997).
Wood, C. E. & Keller-Wood, M. Ontogeny of androgen receptor expression in the ovine fetal central nervous system and pituitary. Neurosci. Lett. 439, 153–156 (2008).
Bouligand, J. et al. Genetics defects in GNRH1: a paradigm of hypothalamic congenital gonadotropin deficiency. Brain Res. 1364, 3–9 (2010).
Teixeira, L. et al. Defective migration of neuroendocrine GnRH cells in human arrhinencephalic conditions. J. Clin. Invest. 120, 3668–3672 (2010).
Grumbach, M. M. & Kaplan, S. L. in Control of the onset of puberty (eds Grumbach, M. M., Sizonenko, P. C., Aubert, M. L.) 1–68 (Williams & Wilkins, Baltimore, 1990).
Schwanzel-Fukuda, M. et al. Migration of luteinizing hormone-releasing hormone (LHRH) neurons in early human embryos. J. Comp. Neurol. 366, 547–557 (1996).
González-Martínez, D., Hu, Y. & Bouloux, P. M. Ontogeny of GnRH and olfactory neuronal systems in man: novel insights from the investigation of inherited forms of Kallmann's syndrome. Front. Neuroendocrinol. 25, 108–130 (2004).
Clark, S. J. et al. Hormone ontogeny in the ovine fetus. XVII. Demonstration of pulsatile luteinizing hormone secretion by the fetal pituitary gland. Endocrinology 115, 1774–1779 (1984).
Liu, L. et al. Effects of pituitary-testicular axis suppression in utero and during the early neonatal period with a long-acting luteinizing hormone-releasing hormone analog on genital development, somatic growth, and bone density in male cynomolgus monkeys in the first 6 months of life. J. Clin. Endocrinol. Metab. 73, 1038–1043 (1991).
Thomas, G. B., McNeilly, A. S., Gibson, F. & Brooks, A. N. Effects of pituitary-gonadal suppression with a gonadotrophin-releasing hormone agonist on fetal gonadotrophin secretion, fetal gonadal development and maternal steroid secretion in the sheep. J. Endocrinol. 141, 317–324 (1994).
Husmann, D. A. Micropenis: an animal model and its human correlates. Adv. Exp. Med. Biol. 511, 41–54 (2002).
Baker, T. G. & Scrimgeour, J. B. Development of the gonad in normal and anencephalic human fetuses. J. Reprod. Fertil. 60, 193–199 (1980).
Cavallo, L. et al. Endocrine function in four anencephalic infants. Horm. Res. 15, 159–166 (1981).
Bouligand, J. et al. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N. Eng. J. Med. 360, 2742–2748 (2009).
Chan, Y. M. et al. GNRH1 mutations in patients with idiopathic hypogonadotropic hypogonadism. Proc. Natl Acad. Sci. USA 106, 11703–11708 (2009).
de Roux, N. et al. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N. Eng. J. Med. 337, 1597–602 (1997).
Caron, P. et al. Resistance of hypogonadic patients with mutated GnRH receptor genes to pulsatile GnRH administration. J. Clin. Endocrinol. Metab. 84, 990–996 (1999).
Pralong, F. P. et al. Complete hypogonadotropic hypogonadism associated with a novel inactivating mutation of the gonadotropin-releasing hormone receptor. J. Clin. Endocrinol. Metab. 84, 3811–3816 (1999).
Söderlund, D., Canto, P., de la Chesnaye, E., Ulloa-Aguirre, A. & Méndez, J. P. A novel homozygous mutation in the second transmembrane domain of the gonadotrophin releasing hormone receptor gene. Clin. Endocrinol. (Oxf.) 54, 493–498 (2001).
Costa, E. M. et al. Two novel mutations in the gonadotropin-releasing hormone receptor gene in Brazilian patients with hypogonadotropic hypogonadism and normal olfaction. J. Clin. Endocrinol. Metab. 86, 2680–2686 (2001).
Layman, L. C., Cohen, D. P., Xie, J. & Smith, G. D. Clinical phenotype and infertility treatment in a male with hypogonadotropic hypogonadism due to mutations Ala129Asp/Arg262Gln of the gonadotropin-releasing hormone receptor. Fertil. Steril. 78, 1317–1320 (2002).
Wolczynski, S. et al. A case of complete hypogonadotropic hypogonadism with a mutation in the gonadotropin-releasing hormone receptor gene. Fertil. Steril. 79, 442–444 (2003).
Karges, B. et al. Mutation Ala(171)Thr stabilizes the gonadotropin-releasing hormone receptor in its inactive conformation, causing familial hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 88, 1873–1879 (2003).
Semple, R. K. et al. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 90, 1849–1855 (2005).
Lanfranco, F. et al. Role of sequence variations of the GnRH receptor and G protein-coupled receptor 54 gene in male idiopathic hypogonadotropic hypogonadism. Eur. J. Endocrinol. 153, 845–852 (2005).
Tenenbaum-Rakover, Y. et al. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J. Clin. Endocrinol. Metab. 92, 1137–1144 (2007).
Teles, M. G. et al. A novel homozygous splice acceptor site mutation of KISS1R in two siblings with normosmic isolated hypogonadotropic hypogonadism. Eur. J. Endocrinol. 163, 29–34 (2010).
Nimri, R. et al. A novel loss-of-function mutation in GPR54/KISS1R leads to hypogonadotropic hypogonadism in a highly consanguineous family. J. Clin. Endocrinol. Metab. 96, E536–E545 (2011).
Topaloglu, A. K. et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat. Genet. 41, 354–358 (2009).
Guran, T. et al. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J. Clin. Endocrinol. Metab. 94, 3633–3639 (2009).
Young, J. et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J. Clin. Endocrinol. Metab. 95, 2287–2295 (2010).
Pitteloud, N. et al. Mutations in fibroblast growth factor receptor 1 cause Kallmann syndrome with a wide spectrum of reproductive phenotypes. Mol. Cell. Endocrinol. 254–255, 60–69 (2006).
Salenave, S. et al. Kallmann's syndrome: a comparison of the reproductive phenotypes in men carrying KAL1 and FGFR1/KAL2 mutations. J. Clin. Endocrinol. Metab. 3, 758–763 (2008).
Gianetti, E. et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J. Clin. Endocrinol. Metab. 95, 2857–2867 (2010).
Hardelin, J. P. et al. Xp22.3 deletions in isolated familial Kallmann's syndrome. J. Clin. Endocrinol. Metab. 76, 827–831 (1993).
Hardelin, J. P. et al. X chromosome-linked Kallmann syndrome: stop mutations validate the candidate gene. Proc. Natl Acad. Sci. USA 89, 8190–8194 (1992).
Meindl, A. et al. Analysis of a terminal Xp22.3 deletion in a patient with six monogenic disorders: implications for the mapping of X linked ocular albinism. J. Med. Genet. 30, 838–842 (1993).
Gu, W. X., Colquhoun-Kerr, J. S., Kopp, P., Bode, H. H. & Jameson, J. L. A novel aminoterminal mutation in the KAL-1 gene in a large pedigree with X-linked Kallmann syndrome. Mol. Genet. Metab. 65, 59–61 (1998).
Weissörtel, R., Strom, T. M., Dörr, H.G., Rauch, A. & Meitinger, T. Analysis of an interstitial deletion in a patient with Kallmann syndrome, X-linked ichthyosis and mental retardation. Clin. Genet. 54, 45–51 (1998).
Nagata, K. et al. A novel interstitial deletion of KAL1 in a Japanese family with Kallmann syndrome. J. Hum. Genet. 45, 237–240 (2000).
Quinton, R. et al. Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clin. Endocrinol. (Oxf.) 55, 163–174 (2001).
Oliveira, L. M. et al. The importance of autosomal genes in Kallmann syndrome: genotype–phenotype correlations and neuroendocrine characteristics. J. Clin. Endocrinol. Metab. 86, 1532–1538 (2001).
Söderlund, D., Canto, P., Méndez, J. P. Identification of three novel mutations in the KAL1 gene in patients with Kallmann syndrome. J. Clin. Endocrinol. Metab. 87, 2589–2592 (2002).
Massin, N. et al. X chromosome-linked Kallmann syndrome: clinical heterogeneity in three siblings carrying an intragenic deletion of the KAL-1 gene. J. Clin. Endocrinol. Metab. 88, 2003–2008 (2003).
Sato, N. et al. Clinical assessment and mutation analysis of Kallmann syndrome 1 (KAL1) and fibroblast growth factor receptor 1 (FGFR1, or KAL2) in five families and 18 sporadic patients. J. Clin. Endocrinol. Metab. 89, 1079–1088 (2004).
Sato, N. et al. Gonadotrophin therapy in Kallmann syndrome caused by heterozygous mutations of the gene for fibroblast growth factor receptor 1: report of three families: case report. Hum. Reprod. 20, 2173–2178 (2005).
Zenaty, D. et al. Paediatric phenotype of Kallmann syndrome due to mutations of fibroblast growth factor receptor 1 (FGFR1). Mol. Cell. Endocrinol. 254–255, 78–83 (2006).
Pitteloud, N. et al. Mutations in fibroblast growth factor receptor 1 cause both Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc. Natl Acad. Sci. USA 103, 6281–6286 (2006).
Sato, N., Ohyama, K., Fukami, M., Okada, M. & Ogata, T. Kallmann syndrome: somatic and germline mutations of the fibroblast growth factor receptor 1 gene in a mother and the son. J. Clin. Endocrinol. Metab. 91, 1415–1418 (2006).
Pitteloud, N. et al. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J. Clin. Invest. 2, 457–463 (2007).
Xu, N. et al. A mutation in the fibroblast growth factor receptor 1 gene causes fully penetrant normosmic isolated hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 92, 1155–1158 (2007).
Falardeau, J. et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J. Clin. Invest. 118, 2822–2831 (2008).
Trarbach, E. B. et al. Nonsense mutations in FGF8 gene causing different degrees of human gonadotropin-releasing deficiency. J. Clin. Endocrinol. Metab. 95, 3491–3496 (2010).
Pitteloud, N. et al. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc. Natl Acad. Sci. USA 104, 17447–17452 (2007).
Cole, L. W. et al. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J. Clin. Endocrinol. Metab. 93, 3551–3559 (2008).
Leroy, C. et al. Biallelic mutations in the prokineticin-2 gene in two sporadic cases of Kallmann syndrome. Eur. J. Hum. Genet. 16, 865–868 (2008).
Abreu, A. P. et al. Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. J. Clin. Endocrinol. Metab. 93, 4113–4118 (2008).
Sarfati, J. et al. A comparative phenotypic study of kallmann syndrome patients carrying monoallelic and biallelic mutations in the prokineticin 2 or prokineticin receptor 2 genes. J. Clin. Endocrinol. Metab. 95, 659–669 (2010).
Kim, H. G. et al. WDR11, a WD protein that interacts with transcription factor EMX1, is mutated in idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am. J. Hum. Genet. 87, 465–479 (2010).
Xu, N. et al. Nasal embryonic LHRH factor (NELF) mutations in patients with normosmic hypogonadotropic hypogonadism and Kallmann syndrome. Fertil. Steril. 95, 1613–1620.e1–e7 (2011).
Reynaud, R. et al. An uncommon phenotype with familial central hypogonadism caused by a novel PROP1 gene mutant truncated in the transactivation domain. J. Clin. Endocrinol. Metab. 90, 4880–4887 (2005).
Weiss, J. et al. Hypogonadism caused by a single amino acid substitution in the β subunit of luteinizing hormone. N. Eng. J. Med. 326, 179–183 (1992).
Valdes-Socin, H. et al. Hypogonadism in a patient with a mutation in the luteinizing hormone β-subunit gene. N. Eng. J. Med. 351, 2619–2625 (2004).
Lofrano-Porto, A. et al. Luteinizing hormone β mutation and hypogonadism in men and women. N. Eng. J. Med. 357, 897–904 (2007).
Bay, K. et al. Insulin-like factor 3 serum levels in 135 normal men and 85 men with testicular disorders: relationship to the luteinizing hormone-testosterone axis. J. Clin. Endocrinol. Metab. 90, 3410–3418 (2005).
Bay, K. et al. Insulin-like factor 3 levels in cord blood and serum from children: effects of age, postnatal hypothalamic-pituitary-gonadal axis activation, and cryptorchidism. J. Clin. Endocrinol. Metab. 92, 4020–4027 (2007).
Young, J. et al. Effects of human recombinant luteinizing hormone and follicle-stimulating hormone in patients with acquired hypogonadotropic hypogonadism: study of Sertoli and Leydig cell secretions and interactions. J. Clin. Endocrinol. Metab. 85, 3239–3244 (2000).
Young, J. et al. Testicular anti-mullerian hormone secretion is stimulated by recombinant human FSH in patients with congenital hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 90, 724–728 (2005).
Tapanainen, J. S., Aittomäki, K., Min, J., Vaskivuo, T. & Huhtaniemi, I. T. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat. Genet. 15, 205–206 (1997).
Lindstedt, G. et al. Follitropin (FSH) deficiency in an infertile male due to FSH beta gene mutation. A syndrome of normal puberty and virilization but underdeveloped testicles with azoospermia, low FSH but high lutropin and normal serum testosterone concentrations. Clin. Chem. Lab. Med. 36, 663–665 (1998).
Layman, L. C. et al. FSH beta gene mutations in a female with partial breast development and a male sibling with normal puberty and azoospermia. J. Clin. Endocrinol. Metab. 87, 3702–3707 (2002).
Winter, J. S., Faiman, C., Hobson, W. C., Prasad, A. V. & Reyes, F. I. Pituitary–gonadal relations in infancy. I. Patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. J. Clin. Endocrinol. Metab. 40, 545–551 (1975).
Waldhauser, F., Weibenssacher, G., Frisch, H. & Pollak, A. Pulsatile secretion of gonadotropins in early infancy. Eur. J. Pediatr. 137, 71–74 (1981).
Müller, J. & Skakkebaek, N. E. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered from sudden death. Int. J. Androl. 6, 143–156 (1983).
Boas, M. et al. Postnatal penile length and growth rate correlate to serum testosterone levels: a longitudinal study of 1,962 normal boys. Eur. J. Endocrinol. 154, 125–129 (2006).
Andersson, A. et al. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J. Clin. Endocrinol. Metab. 83, 675–681 (1998).
Grumbach, M. M. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J. Clin. Endocrinol. Metab. 90, 3122–3127 (2005).
Bergadá, I. et al. Time course of the serum gonadotropin surge, inhibins, and anti-Müllerian hormone in normal newborn males during the first month of life. J. Clin. Endocrinol. Metab. 91, 4092–4098 (2006).
Cortes, D., Müller, J. & Skakkebaeck, N. E. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int. J. Androl. 10, 589–596 (1987).
Sharpe, R. M. et al. Role of the neonatal period of pituitary-testicular activity in germ cell proliferation and differentiation in the primate testis. Hum. Reprod. 18, 2110–2117 (2003).
Berensztein, E. B., Sciara, M. I., Rivarola, M. A. & Belgorosky, A. Apoptosis and proliferation of human testicular somatic and germ cells during prepuberty: high rate of testicular growth in newborns mediated by decreased apoptosis. J. Clin. Endocrinol. Metab. 87, 5113–5118 (2002).
Rey, R. A., Musse, M., Venara, M. & Chemes, H. E. Ontogeny of the androgen receptor expression in the fetal and postnatal testis: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc. Res. Tech. 72, 787–795 (2009).
Evain-Brion, D., Gendrel, D., Bozzola, M., Chaussain, J. L. & Job, J. C. Diagnosis of Kallmann's syndrome in early infancy. Acta Paediatr. Scand. 71, 937–940 (1982).
Kaplan, J. D., Bernstein, J. A., Kwan, A. & Hudgins, L. Clues to an early diagnosis of Kallmann syndrome. Am. J. Med. Genet. A 152A, 2796–2801 (2010).
Pitteloud, N. et al. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 87, 4128–4136 (2002).
Coutant, R. et al. Baseline inhibin B and anti-Mullerian hormone measurements for diagnosis of hypogonadotropic hypogonadism (HH) in boys with delayed puberty. J. Clin. Endocrinol. Metab. 95, 5225–5232 (2010).
Delemarre-van de Waal, H. A. Application of gonadotropin releasing hormone in hypogonadotropic hypogonadism-diagnostic and therapeutic aspects. Eur. J. Endocrinol. 151 (Suppl. 3), U89–U94 (2004).
Delemarre, E. M., Felius, B. & Delemarre-van de Waal, H. A. Inducing puberty. Eur. J. Endocrinol. 159 (Suppl. 1), S9–S15 (2008).
Richmond, E. J. & Rogol, A. D. Male pubertal development and the role of androgen therapy. Nat. Clin. Pract. Endocrinol. Metab. 3, 338–344 (2007).
Trabado, S. et al. Estradiol levels in men with congenital hypogonadotropic hypogonadism and the effects of different modalities of hormonal treatment. Fertil. Steril. 95, 2324–2329.e1–e3 (2011).
Cunningham, G. R. & Toma, S. M. Clinical review: Why is androgen replacement in males controversial? J. Clin. Endocrinol. Metab. 96, 38–52 (2011).
Bin-Abbas, B., Conte, F. A., Grumbach, M. M. & Kaplan, S. L. Congenital hypogonadotropic hypogonadism and micropenis: effect of testosterone treatment on adult penile size why sex reversal is not indicated. J. Pediatr. 134, 579–583 (1999).
Bouvattier, C., Mignot, B., Lefèvre, H., Morel, Y. & Bougnères, P. Impaired sexual activity in male adults with partial androgen insensitivity. J. Clin. Endocrinol. Metab. 91, 3310–3315 (2006).
Schaison, G., Young, J., Pholsena, M., Nahoul, K. & Couzinet, B. Failure of combined follicle-stimulating hormone-testosterone administration to initiate and/or maintain spermatogenesis in men with hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 77, 1545–1549 (1993).
Sinisi, A. A. et al. Efficacy of recombinant human follicle stimulating hormone at low doses in inducing spermatogenesis and fertility in hypogonadotropic hypogonadism. J. Endocrinol. Invest. 33, 618–623 (2010).
Matsumoto, A. M. Hormonal therapy of male hypogonadism. Endocrinol. Metab. Clin. North Am. 23, 857–875 (1994).
Finkel, D. M., Phillips, J. L. & Snyder, P. J. Stimulation of spermatogenesis by gonadotropins in men with hypogonadotropic hypogonadism. N. Eng. J. Med. 313, 651–655 (1985).
Burqués, S. & Calderòn, M. D. Subcutaneous self-administration of highly purified follicle stimulating hormone and human chorionic gonadotrophin for the treatment of male hypogonadotrophic hypogonadism. Spanish Collaborative Group on Male Hypogonadotropic Hypogonadism. Hum. Reprod. 12, 980–986 (1997).
Burris, A. S., Clark, R. V., Vantman, D. J. & Sherins, R. J. A low sperm concentration does not preclude fertility in men with isolated hypogonadotropic hypogonadism after gonadotropin therapy. Fertil. Steril. 50, 343–347 (1988).
Büchter, D., Behre, H. M., Kliesch, S. & Nieschlag, E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur. J. Endocrinol. 139, 298–303 (1998).
Saal, W., Happ, J., Cordes, U., Baum, R. P. & Schmidt, M. Subcutaneous gonadotropin therapy in male patients with hypogonadotropic hypogonadism. Fertil. Steril. 56, 319–324 (1991).
Kung, A. W., Zhong, Y. Y., Lam, K. S. & Wang, C. Induction of spermatogenesis with gonadotrophins in Chinese men with hypogonadotrophic hypogonadism. Int. J. Androl 17, 241–247 (1994).
Liu, P. Y. et al. Efficacy and safety of recombinant human follicle stimulating hormone (Gonal-F) with urinary human chorionic gonadotrophin for induction of spermatogenesis and fertility in gonadotrophin-deficient men. Hum. Reprod. 14, 1540–1545 (1999).
Liu, P. Y. et al. Predicting pregnancy and spermatogenesis by survival analysis during gonadotrophin treatment of gonadotrophin-deficient infertile men. Hum. Reprod. 17, 625–633 (2002).
Burger, H. G. & Baker, H. W. Therapeutic considerations and results of gonadotropin treatment in male hypogonadotropic hypogonadism. Ann. NY Acad. Sci. 438, 447–453 (1984).
Hayes, F. J., Seminara, S. B. & Crowley, W. F. Jr. Hypogonadotropic hypogonadism. Endocrinol. Metab. Clin. North Am. 27, 739–763 (1998).
Vicari, E. et al. Therapy with human chorionic gonadotropin alone induces spermatogenesis in men with isolated hypogonadotrophic hypogonadism-long-term follow-up. Int. J. Androl. 15, 320–329 (1992).
Matsumoto, A. M., Karpas, A. E. & Bremner, W. J. Chronic human chorionic gonadotropin administration in normal men: evidence that follicle-stimulating hormone is necessary for the maintenance of quantitatively normal spermatogenesis in man. J. Clin. Endocrinol. Metab. 62, 1184–1192 (1986).
Okuyama, A. et al. Testicular responsiveness to long-term administration of hCG and hMG in patients with hypogonadotropic hypogonadism. Horm. Res. 23, 21–30 (1986).
Kirk, J. M., Savage, M. O., Grant, D. B., Bouloux, P. M. & Besser, G. M. Gonadal function and response to human chorionic and menopausal gonadotrophic therapy in male patients with idiopathic hypogonadotrophic hypogonadism. Clin. Endocrinol. (Oxf.) 41, 57–63 (1994).
[No authors listed] Efficacy and safety of highly purified urinary follicle-stimulating hormone with human chorionic gonadotropin for treating men with isolated hypogonadotropic hypogonadism. European Metrodin HP Study Group. Fertil. Steril. 70, 256–262 (1998).
Schopohl, J., Mehltretter, G., von Zumbusch, R., Eversmann, T. & von Werder, K. Comparison of gonadotropin-releasing hormone and gonadotropin therapy in male patients with idiopathic hypothalamic hypogonadism. Fertil. Steril. 56, 1143–1150 (1991).
Kliesch, S., Behre, H. M. & Nieschlag, E. Recombinant human follicle-stimulating hormone and human chorionic gonadotropin for induction of spermatogenesis in a hypogonadotropic male. Fertil. Steril. 63, 1326–1328 (1995).
Bouloux, P., Warne, D. W. & Loumaye, E. Efficacy and safety of recombinant human follicle-stimulating hormone in men with isolated hypogonadotropic hypogonadism. Fertil. Steril. 77, 270–273 (2002).
Bouloux, P. M. et al. Induction of spermatogenesis by recombinant follicle-stimulating hormone (puregon) in hypogonadotropic azoospermic men who failed to respond to human chorionic gonadotropin alone. J. Androl. 24, 604–611 (2003).
Warne, D. W. et al. A combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropin. Fertil. Steril. 2, 594–604 (2009).
Liu, P. Y. et al. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J. Clin. Endocrinol. Metab. 94, 801–808 (2009).
Oldereid, N. B., Abyholm, T. & Tanbo, T. G. Spermatogenesis and fertility outcome in male hypogonadotrophic hypogonadism. Hum. Fertil. (Camb.) 13, 83–89 (2010).
Nane, I. et al. Primary gonadotropin releasing hormone and adjunctive human chorionic gonadotropin treatment in cryptorchidism: a clinical trial. Urology 49, 108–111 (1997).
Ritzén, E. M. Undescended testes: a consensus on management. Eur. J. Endocrinol. 159 (Suppl. 1), S87–S90 (2008).
Aksglaede, L. et al. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum. Reprod. Update. 12, 39–48 (2006).
Resorlu, B., Abdulmajed, M. I., Kara, C., Unsal, A. & Aydos, K. Is intracytoplasmic sperm injection essential for the treatment of hypogonadotrophic hypogonadism? A comparison between idiopathic and secondary hypogonadotrophic hypogonadism. Hum. Fertil. (Camb.) 12, 204–208 (2009).
Frapsauce, C. et al. Birth after TESE-ICSI in a man with hypogonadotropic hypogonadism and congenital adrenal hypoplasia linked to a DAX-1 (NR0B1) mutation. Hum. Reprod. 26, 724–728 (2011).
Raivio, T., Toppari, J., Perheentupa, A., McNeilly, A. S. & Dunkel, L. Treatment of prepubertal gonadotrophin-deficient boys with recombinant human follicle-stimulating hormone. Lancet 350, 263–264 (1997).
Bouvattier, C., Tauber, M., Jouret, B., Chaussain, J. L. & Rochiccioli, P. Gonadotropin treatment of hypogonadotropic hypogonadal adolescents. J. Pediatr. Endocrinol. Metab. 12, 339–344 (1999).
Raivio, T. Wikström, A. M. & Dunkel, L. Treatment of gonadotropin-deficient boys with recombinant human FSH: long-term observation and outcome. Eur. J. Endocrinol. 156, 105–111 (2007).
Main, K. M., Schmidt, I. M., Toppari, J. & Skakkebaek, N. E. Early postnatal treatment of hypogonadotropic hypogonadism with recombinant human FSH and LH. Eur. J. Endocrinol. 146, 75–79 (2002).
Main, K. M., Schmidt, I. M. & Skakkebaek, N. E. A possible role for reproductive hormones in newborn boys: progressive hypogonadism without the postnatal testosterone peak. J. Clin. Endocrinol. Metab. 85, 4905–4907 (2000).
Bougnères, P. et al. Effects of an early postnatal treatment of hypogonadotropic hypogonadism with a continuous subcutaneous infusion of recombinant follicle-stimulating hormone and luteinizing hormone. J. Clin. Endocrinol. Metab. 93, 2202–2205 (2008).
Christiansen, P. et al. Treatment of cryptorchidism with human chorionic gonadotropin or gonadotropin releasing hormone. A double-blind controlled study of 243 boys. Horm. Res. 30, 187–192 (1988).
Dunkel, L., Taskinen, S., Hovatta, O., Tilly, J. L. & Wikström, S. Germ cell apoptosis after treatment of cryptorchidism with human chorionic gonadotropin is associated with impaired reproductive function in the adult. J. Clin. Invest. 100, 2341–2346 (1997).
Kaleva, M. & Toppari, J. Cryptorchidism: an indicator of testicular dysgenesis? Cell Tissue Res. 322, 167–172 (2005).
Ramaswamy, S., Plant, T. M. & Marshall, G. R. Pulsatile stimulation with recombinant single chain human luteinizing hormone elicits precocious sertoli cell proliferation in the juvenile male rhesus monkey (Macaca mulatta). Biol. Reprod. 63, 82–88 (2000).
Acknowledgements
This work was supported by grants from Université Paris-Sud (Bonus Qualité Recherche), Institut National de la Santé et de la Recherche Médicale (INSERM), Agence Nationale de la Recherche Genopath (ANR KALGENOPATH), Fondation pour la Recherche Médicale (FRM), Programme Hospitalier de Recherche Clinique (PHRC National: Hypo-Protéo) and Agence Française de Lutte contre le Dopage (AFLD).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article and provided a substantial contribution to discussions of the content. C. Bouvattier, L. Maione and J. Young contributed equally to writing the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bouvattier, C., Maione, L., Bouligand, J. et al. Neonatal gonadotropin therapy in male congenital hypogonadotropic hypogonadism. Nat Rev Endocrinol 8, 172–182 (2012). https://doi.org/10.1038/nrendo.2011.164
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2011.164
This article is cited by
-
Successful pregnancy and delivery after ovulation induction therapy in a woman with congenital hypogonadotropic hypogonadism: a case report
BMC Pregnancy and Childbirth (2023)
-
Genetische und epigenetische Einflüsse auf den Pubertätsverlauf in Bezug auf Pubertas praecox vera und Pubertas tarda
Gynäkologische Endokrinologie (2023)
-
Aktualisierte Handlungsempfehlung nach der S1-Leitlinie zu Pubertas tarda und Hypogonadismus
Monatsschrift Kinderheilkunde (2023)
-
Testosterone therapy in children and adolescents: to whom, how, when?
International Journal of Impotence Research (2022)
-
Pathogenic mosaic variants in congenital hypogonadotropic hypogonadism
Genetics in Medicine (2020)