Abstract
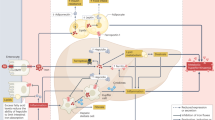
Hereditary hemochromatosis (HH) is a genetic condition that can lead to unregulated absorption of iron from the gut with resultant iron overload. The most common form of HH is caused by mutations in the HFE gene, with most cases of HH presenting in patients who are homozygous for the Cys282Tyr mutation. The prevalence of HFE gene mutations in persons of Northern European ancestry is fairly high (0.3–0.7% homozygous and 9–14% heterozygous for the Cys282Tyr mutation), but the penetrance of the disease is considered fairly low and is quite variable. While routine screening of the general population is not recommended, a targeted approach to screening in symptomatic patients and in those with a family member with iron overload is warranted. Untreated, iron overload can lead to considerable morbidity including liver cirrhosis, arthritis and diabetes mellitus, and increased mortality. The pathophysiology of diabetes mellitus in HH is thought to be due primarily to defects in the early insulin response to glucose. An Hfe−/− mouse model of HH has demonstrated defects in β-cell function and β-cell apoptosis that may be mediated by increased oxidative stress. Fortunately, these defects seem to be reversible if phlebotomy treatment is initiated before the development of cirrhosis or diabetes mellitus in patients. Further research into the long-term effects of treatment on prevention of diabetes mellitus in HH is needed.
Key Points
-
Most cases of hereditary hemochromatosis (HH) result from mutations in the HFE gene, consequential low hepcidin levels and continued iron absorption from the gut despite high iron levels
-
Homozygosity for the Cys282Tyr HFE gene mutation occurs in 0.3–0.7% of people with North European ancestry, but clinical disease occurs in only a small percentage of these
-
Pathophysiology of diabetes mellitus in iron overload is thought to be a result of β-cell dysfunction that leads to inadequate insulin secretion for the prevailing degree of insulin sensitivity
-
Increased oxidative stress may be one mechanism whereby iron overload causes β-cell dysfunction and β-cell apoptosis
-
Early identification and treatment with phlebotomy before the onset of diabetes mellitus and cirrhosis can prevent the increased morbidity and mortality associated with HH
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nemeth, E. et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 (2004).
Adams, P. C. & Barton, J. C. Haemochromatosis. Lancet 370, 1855–1860 (2007).
Pietrangelo, A. Hemochromatosis: an endocrine liver disease. Hepatology 46, 1291–1301 (2007).
Bridle, K. R. et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homeostasis. Lancet 361, 669–673 (2003).
Morrison, E. D. et al. Serum ferritin level predicts advanced hepatic fibrosis among U. S. patients with phenotypic hemochromatosis. Ann. Intern. Med. 138, 627–633 (2003).
Mura, C., Raguenes, O. & Férec, C. HFE mutations analysis in 711 hemochromatosis probands: evidence for S65C implication in mild form of hemochromatosis. Blood 93, 2502–2505 (1999).
Papanikolaou, G. et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat. Genet. 36, 77–82 (2004).
Lee, P. L., Beutler, E., Rao, S. V. & Barton, J. C. Genetic abnormalities and juvenile hemochromatosis: mutations of the HJV gene encoding hemojuvelin. Blood 103, 4669–4671 (2004).
Roetto, A. et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat. Genet. 33, 21–22 (2003).
Camaschella, C. et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat. Genet. 25, 14–15 (2000).
Njajou, O. T. et al. A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat. Genet. 28, 213–214 (2001).
Andersen, R. V., Tybjaerg-Hansen, A., Appleyard, M., Birgens, H. & Nordestgaard, B. G. Hemochromatosis mutations in the general population: iron overload progression rate. Blood 103, 2914–2919 (2004).
Asberg, A. et al. Screening for hemochromatosis: high prevalence and low morbidity in an unselected population of 65,238 persons. Scand. J. Gastroenterol. 36, 1108–1115 (2001).
Olynyk, J. K. et al. A population-based study of the clinical expression of the hemochromatosis gene. N. Engl. J. Med. 341, 718–724 (1999).
Deugnier, Y. et al. Gender-specific phenotypic expression and screening strategies in C282Y-linked haemochromatosis: a study of 9396 French people. Br. J. Haematol. 118, 1170–1178 (2002).
Beutler, E., Felitti, V. J., Koziol, J. A., Ho, N. J. & Gelbart, T. Penetrance of 845G--> A (C282Y) HFE hereditary haemochromatosis mutation in the U. S. A. Lancet 359, 211–218 (2002).
Allen, K. J. et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N. Engl. J. Med. 358, 221–230 (2008).
Gordeuk, V. R. et al. Serum ferritin concentrations and body iron stores in a multicenter, multiethnic primary-care population. Am. J. Hematol. 83, 618–626 (2008).
Gurrin, L. C. et al. The natural history of serum iron indices for HFE C282Y homozygosity associated with hereditary hemochromatosis. Gastroenterology 135, 1945–1952 (2008).
Gurrin, L. C. et al. HFE C282Y/H263D compound heterozygotes are at low risk of hemochromatosis-related morbidity. Hepatology 50, 94–101 (2009).
Niederau, C. et al. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N. Engl. J. Med. 313, 1256–1262 (1985).
Whitlock, E. P., Garlitz, B. A., Harris, E. L., Beil, T. L. & Smith, P. R. Screening for hereditary hemochromatosis: a systematic review for the U. S. Preventive Services Task Force. Ann. Intern. Med. 145, 209–223 (2006).
Powell, L. W. et al. Screening for hemochromatosis in asymptomatic subjects with or without a family history. Arch. Intern. Med. 166, 294–301 (2006).
Olynyk, J. K., Hagan, S. E., Cullen, D. J., Beilby, J. & Whittall, D. E. Evolution of untreated hereditary hemochromatosis in the Busselton population: a 17-year study. Mayo Clin. Proc. 79, 309–313 (2004).
Williams, R., Smith, P. M., Spicer, E. J., Barry, M. & Sherlock, S. Venesection therapy in idiopathic haemochromatosis. An analysis of 40 treated and 18 untreated patients. Q. J. Med. 38, 1–16 (1969).
Adams, P. C., Kertesz, A. E. & Valberg, L. S. Clinical presentation of hemochromatosis: a changing scene. Am. J. Med. 90, 445–449 (1991).
Bacon, B. R. & Sadiq, S. A. Hereditary hemochromatosis: presentation and diagnosis in the 1990s. Am. J. Gastroenterol. 92, 784–789 (1997).
Bulaj, Z. J. et al. Disease-related conditions in relatives of patients with hemochromatosis. N. Engl. J. Med. 343, 1529–1535 (2000).
Swinkels, D. W., Jorna, A. T. & Raymakers, R. A. Synopsis of the Dutch multidisciplinary guideline for the diagnosis and treatment of hereditary haemochromatosis. Neth. J. Med. 65, 452–455 (2007).
Olynyk, J. K., Gan, E. & Tan, T. Predicting iron overload in hyperferritinemia. Clin. Gastroenterol. Hepatol. 7, 359–362 (2009).
St Pierre, T. G., Clark, P. R. & Chua-Anusorn, W. Measurement and mapping of liver iron concentrations using magnetic resonance imaging. Ann. NY Acad. Sci. 1054, 379–385 (2005).
Burke, W., Reyes, M. & Imperatore, G. Hereditary haemochromatosis: a realistic approach to prevention of iron overload disease in the population. Best Prac. & Res. Clin. Haematol. 15, 315–328 (2002).
Bradley, L. A., Haddow, J. E. & Palomaki, G. E. Population screening for haemochromatosis: a unifying analysis of published intervention trials. J. Med. Screen. 3, 178–184 (1996).
U. S. Preventative Services Task Force. Screening for hemochromatosis: recommendation statement. Ann. Intern. Med. 145, 204–208 (2006).
Phatak, P. D., Bonkovsky, H. L. & Kowdley, K. V. Hereditary hemochromatosis: time for targeted screening. Ann. Intern. Med. 149, 270–272 (2008).
Cadet, E. et al. A targeted approach significantly increases the identification rate of patients with undiagnosed haemochromatosis. J. Intern. Med. 253, 217–224 (2003).
Tavill, A. S. Diagnosis and management of hemochromatosis. Hepatology 33, 1321–1328 (2001).
Worwood, M. Inherited iron loading: genetic testing in diagnosis and management. Blood Reviews 19, 69–88 (2005).
Niederau, C. et al. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology 110, 1107–1119 (1996).
Fargion, S. et al. Survival and prognostic factors in 212 Italian patients with genetic hemochromatosis. Hepatology 15, 655–659 (1992).
Adams, P. C., Speechley, M. & Kertesz, A. E. Long-term survival analysis in hereditary hemochromatosis. Gastroenterology 101, 368–372 (1991).
McClain, D. A. et al. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia 49, 1661–1669 (2006).
Dymock, I. W., Cassar, J., Pyke, D. A., Oakley, W. G. & Williams, R. Observations on the pathogenesis, complications and treatment of diabetes in 115 cases of haemochromatosis. Am. J. Med. 52, 203–210 (1972).
O'Sullivan, E. P., McDermott, J. H., Murphy, M. S., Sen, S. & Walsh, C. H. Declining prevalence of diabetes mellitus in hereditary haemochromatosis--the result of earlier diagnosis. Diabetes Res. Clin. Prac. 81, 316–320 (2008).
Davis, T. M. et al. Prevalence, characteristics, and prognostic significance of HFE gene mutations in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care 31, 1795–1801 (2008).
Ellervik, C. et al. Prevalence of hereditary haemochromatosis in late-onset type 1 diabetes mellitus: a retrospective study. Lancet 358, 1405–1409 (2001).
Salonen, J. T., Tuomainen, T. P. & Kontula, K. Role of C282Y mutation in haemochromatosis gene in development of type 2 diabetes in healthy men: prospective cohort study. BMJ 320, 1706–1707 (2000).
Halsall, D. J., McFarlane, I., Luan, J., Cox, T. M. & Wareham, N. J. Typical type 2 diabetes mellitus and HFE gene mutations: a population-based case–control study. Hum. Mol. Genet. 12, 1361–1365 (2003).
Cavallo-Perin, P. et al. Insulin resistance and hyperinsulinemia in homozygous beta-thalassemia. Metabolism 44, 281–286 (1995).
Merkel, P. A. et al. Insulin resistance and hyperinsulinemia in patients with thalassemia major treated by hypertransfusion. N. Engl. J. Med. 318, 809–814 (1988).
Dmochowski, K., Finegood, D. T., Francombe, W., Tyler, B. & Zinman, B. Factors determining glucose tolerance in patients with thalassemia major. J. Clin. Endocrinol. Metab. 77, 478–483 (1993).
Hramiak, I. M., Finegood, D. T. & Adams, P. C. Factors affecting glucose tolerance in hereditary hemochromatosis. Clin. Invest. Med. 20, 110–118 (1997).
Cooksey, R. C. et al. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 145, 5305–5312 (2004).
Cighetti, G. et al. Oxidative status and malondialdehyde in beta-thalassaemia patients. Eur. J. Clin. Invest. 32 (Suppl. 1), 55–60 (2002).
Gutteridge, J. M., Rowley, D. A., Griffiths, E. & Halliwell, B. Low-molecular-weight iron complexes and oxygen radical reactions in idiopathic haemochromatosis. Clin. Sci. (Lond.) 68, 463–467 (1985).
Jouihan, H. A. et al. Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Mol. Med. 14, 98–108 (2008).
Abraham, D., Rogers, J., Gault, P., Kushner, J. P. & McClain, D. A. Increased insulin secretory capacity but decreased insulin sensitivity after correction of iron overload by phlebotomy in hereditary haemochromatosis. Diabetologia 49, 2546–2551 (2006).
Acknowledgements
This work is supported in part by the VA Puget Sound Health Care System, Seattle, WA and the Department of Veterans Affairs (to K. M. U.) and NIH Grant DK02957 (to K. V. K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Utzschneider, K., Kowdley, K. Hereditary hemochromatosis and diabetes mellitus: implications for clinical practice. Nat Rev Endocrinol 6, 26–33 (2010). https://doi.org/10.1038/nrendo.2009.241
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.241
This article is cited by
-
Prenatal iron exposure and childhood type 1 diabetes
Scientific Reports (2018)
-
The mechanisms of systemic iron homeostasis and etiology, diagnosis, and treatment of hereditary hemochromatosis
International Journal of Hematology (2018)
-
Serum ferritin and obstructive sleep apnea—epidemiological study
Sleep and Breathing (2018)
-
Diabetes mellitus Typ 3c – Prävalenz, Diagnose, Besonderheiten der Therapie
Der Diabetologe (2018)
-
Type 2 Diabetes and Hepatocellular Carcinoma: Risk Factors and Pathogenesis
Current Diabetes Reports (2017)