Cancer immune-cell therapies are increasing in prominence as breakthrough treatments. Since 2017, several chimeric antigen receptor (CAR) T-cell therapies have been approved and have demonstrated excellent therapeutic efficacy in haematologic malignancies. All of the currently approved CAR T-cell therapies are patient-derived, and they face two major challenges: variability in the quality and quantity of produced cells; and insufficient efficacy due to cell exhaustion. The potential use of allogeneic induced pluripotent stem cell (iPSC)-derived T cells (iPS-Ts) is therefore attracting attention for the stable production and rejuvenation of manufactured immune cells.
Founded in 2015, Thyas is a Kyoto University spinoff whose research and development is focused on the clinical application of iPSC-derived immune cells, based on the research advances made by Shin Kaneko at the Centre for iPS Cell Research and Application (CiRA) in Japan. In 2013, Kaneko — the founder of Thyas — was the first scientist to accomplish T-cell differentiation from human iPSCs, and to demonstrate in vitro and in vivo efficacy. Thyas is pioneering immune-cell therapies using iPSCs as an allogeneic source for the treatment of oncological and non-oncological clinical indications. Its platform technologies enable Thyas to consistently produce energized immune cells, especially cluster of differentiation 8 (CD8)-α and CD8-β T cells, for clinical studies (Fig. 1).
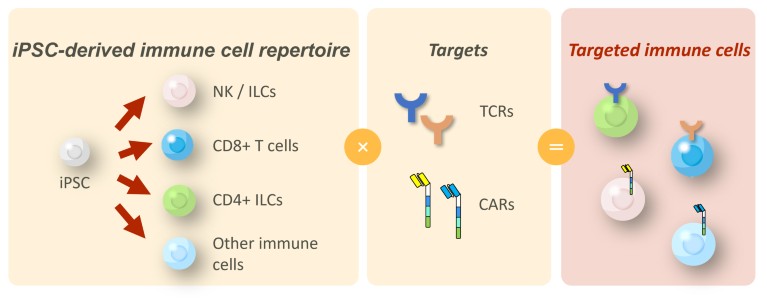
Figure 1. Thyas’ proprietary induced pluripotent stem cell (iPSC)-derived immune-cell platform. The core technology utilizes the established differentiation methods of various types of immune cell from iPSCs, including natural killer (NK) cells, killer T cells, and cluster of differentiation (CD)4-expressing innate lymphoid cells (ILCs). Combined with chimeric antigen receptors (CARs) or T-cell receptors (TCRs) of interest, this platform can generate therapeutic immune cells for many types of cancer.
Creation of antigen-specific cytotoxic T cells
For iPS-Ts to acquire antigen-specific cytotoxicity, the induction of CD8-α/β expression is required for strong avidity between the T-cell receptor (TCR) and human leukocyte antigen (HLA)/peptides (cancer antigens). However, it is difficult to produce T cells that express CD8-α/β from iPSCs. Kaneko’s group demonstrated that they could produce functional iPS-Ts with CD8-α/β expression with appropriate in vitro and in vivo efficacy1. CD8-α/β heterodimer T cells are deemed to have better migratory capacity into tumour sites. Thyas has optimized Kaneko’s method for clinical production and will initiate clinical trials soon.
Feeder-free production systems
In addition to the inherent difficulty of inducing CD8-α/β T cells, conventional T-cell differentiation processes that use stromal or feeder cells carry a risk of pathogen contamination, may result in unstable production, and involve additional costs associated with the use of peripheral blood mononuclear cells (PBMCs) and the various tests required to check for purity. Kaneko’s group demonstrated a reliable large-scale feeder-free production system by culturing iPSC-derived hematopoietic stem cells (HSCs) in the presence of cytokine cocktails that activate Notch signalling and cell adhesion. The iPS-Ts modified in this system exhibited early-memory phenotypes, including high replicative capacity and an ability to give rise to potent effector cells2,3. Moreover, Thyas has further optimized and established a feeder-free T-cell culture system for clinical-scale production, and begun Good Manufacturing Practice (GMP) grade production of TCR iPS-Ts for clinical trials.
Hypoimmunogenic T cells
For iPSC-derived immune cells to be used repeatedly without HLA matching, they must evade rejection from the host’s immune system. Thyas is taking a comprehensive approach to creating hypoimmunogenic iPSCs as a source of clinical materials, which is expected to be the next standard for cancer immune-cell therapy. In 2021, Kaneko’s group produced iPS-Ts from genetically modified hypoimmunogenic iPSCs and demonstrated persistence of iPS-Ts in the presence of allogeneic PBMCs, as well as in vivo anti-tumour activity4. Thyas’ efforts to adopt hypoimmunogenic iPSCs as a source for next-generation products are ongoing.
Unlocking the potential of cancer immune-cell therapies
Cancer immune-cell therapy using iPS-Ts has the potential to become a curative intervention for the treatment not only of myeloproliferative diseases but also of solid tumours. While the clinical development of natural killer (NK) cells from iPSCs is currently leading this field, iPS-Ts are expected to drive the next wave of iPSC-derived immune-cell applications. Founded in one of the first centres of iPSC research, Thyas is becoming a leader of iPSC-derived cancer immune-cell therapy.

