Despite numerous treatment advances, chronic hepatitis B virus (HBV) infection remains a largely incurable disease impacting more than 290 million people worldwide. All patients with chronic HBV face an elevated risk of death and more than 800,000 people die each year from HBV-related complications, primarily cirrhosis and liver cancers.
A major treatment advance occurred with the introduction of potent antivirals with a high barrier to resistance. In some cases, and over years of use, these drugs can reduce or even reverse progressive liver fibrosis1. Unfortunately, these suppressive therapies rarely achieve a functional cure, defined as sustained control of the virus after stopping therapy.
Vir Biotechnology, a commercial-stage immunology company focused on combining immunologic insights with cutting-edge technologies to treat and prevent serious infectious diseases, is tackling the unmet need for a potential HBV functional cure. Herein, we present Vir’s two-pronged approach and highlight some key remaining questions.
A path to new treatments
HBV’s morbidity arises in part from its ‘stealth’ ability to replicate and spread. The majority of patients are asymptomatic during the early and chronic stages of infection even as the virus, one of the smallest DNA viruses in existence, enters susceptible liver cells — known as hepatocytes — and begins replicating.
Inside the hepatocytes, HBV produces a surplus of viral antigens, including hepatitis B e antigen (HBeAg) and hepatitis B s antigen (HBsAg), which is HBV’s envelope protein. In addition to the excess HBsAg present in circulating virions, large amounts of subviral particles containing HBsAg are secreted by infected cells. Intracellular antigens, as well as the extracellular antigens found in the combination of virions and subviral particles, are believed to negatively impact B and T cell responses2, exhaust the immune system and thus prevent HBV clearance by the endogenous immune response3. With ongoing chronic infection, a patient’s risk of liver fibrosis increases over time, elevating the risk of cirrhosis or liver cancer. Meanwhile, the predominant absence of symptoms both prevents early treatment and increases the possibility of further transmission.
It is notable that even in HBV patients who have achieved a functional cure, chronic HBV can be reactivated by suppressing the immune system4.
We hypothesize that reducing antigen levels to encourage an immune system response and suppressing viral replication to protect healthy hepatocytes may result in a functional cure. This is the scientific rationale behind Vir’s two-pronged approach.
Specifically, Vir is evaluating multiple investigational, combination therapies as part of its broad clinical programme.
• VIR-2218, the foundational candidate, is a subcutaneously administered GalNAc-conjugated small interfering ribonucleic acid (siRNA) developed with Alnylam Pharmaceuticals, Inc. VIR-2218 incorporates Alnylam’s ‘Enhanced Stabilization Chemistry Plus’ technology to improve specificity while maintaining metabolic stability for in vivo activity5. VIR-2218 has also been designed to target HBV’s X gene region, shared by all HBV RNAs, for broader activity (Fig. 1).
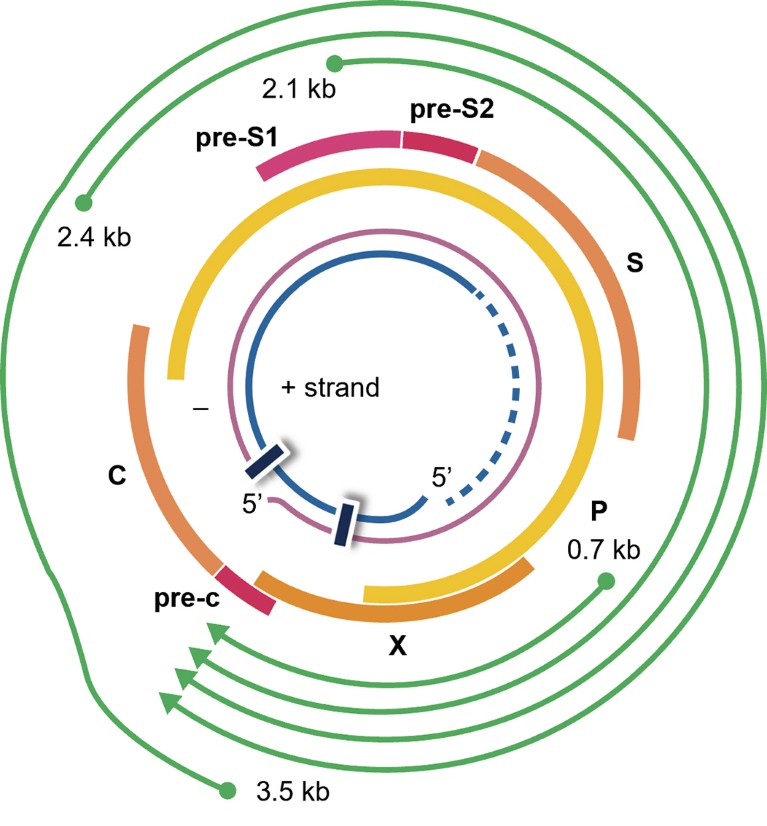
Figure 1. VIR-2218 targets the HBx region of the HBV genome to silence HBV transcripts.
Preliminary results, not yet peer-reviewed, from a phase II clinical trial, suggest that VIR-2218 elicited dose-dependent reductions in circulating HBsAg through 48 weeks (NCT03672188)6.
We believe the potency of immunomodulating therapy will be enhanced — the second part of our dual approach — by this intracellular and extracellular antigen supression. Thus, we are testing several immunomodulatory agents with distinct mechanisms of action to determine which may provide benefit when paired with VIR-2218.
• VIR-2218 with VIR-3434: VIR-3434 is a subcutaneously administered antibody (Fig. 2). Preliminary preclinical data, not yet peer-reviewed, suggest that it neutralizes HBV, blocks entry into hepatocytes and reduces the level of virions and HBsAg in circulation. Additionally, it has been engineered to stimulate dendritic cell maturation and to therefore act as a therapeutic T cell vaccine (NCT04423393, NCT04856085)7.
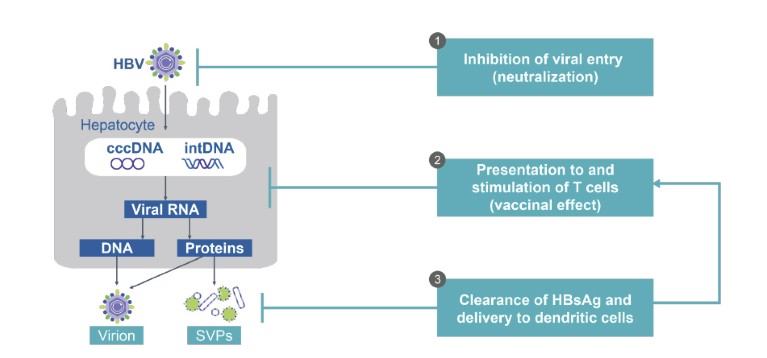
Figure 2. VIR-3434: an Fc-engineered human antibody against hepatitis B s antigen with multiple potential mechanisms of action. cccDNA, covalently closed circular DNA; DNA, deoxyribonucleic acid; HBsAg, hepatitis B s antigen; HBV, hepatitis B virus; intDNA, integrated DNA; RNA, ribonucleic acid; SVPs, subviral particles.
• VIR-2218 with PEG-IFN-α: PEG-IFN-α is currently the only approved treatment shown to achieve a functional cure, albeit at very low rates8. Vir is conducting clinical trials to evaluate whether VIR-2218 will enhance the rate of functional cure while shortening the duration of therapy (NCT04412863, NCT04856085).
• VIR-2218 with a TLR8 agonist and PD-1 inhibitor: TLR8 agonists and PD-1 inhibitors are each being evaluated as monotherapy to determine their immunomodulatory activity against HBV. Vir is evaluating VIR-2218 with the TLR-8 agonist selgantolimod (GS-9688) and nivolumab, a PD-1 inhibitor, in antiviral-suppressed patients and viremic patients (NCT04891770).
• VIR-2218+BRII-179: BRII-179 is an investigational vaccine intended to enhance both B cell and T cell immunity. A study evaluating this combination approach is being conducted by our partner, Brii Biosciences (NCT04749368).
Hope for the future
Critically, investments made by governments, academia and industry towards a potential functional cure have accelerated in the past decade, especially since the remarkable achievement of oral cures for chronic hepatitis C. However, many questions about HBV’s immunology, which we believe is key to achieving a functional HBV cure, remain.
Deeper insights into the degree of antigen reduction required for an appropriate immune response, the optimal type of immunostimulation, as well as the cellular process for removing infected hepatocytes, including the degree and duration of CD4 T cell and B cell stimulation, could expand immunomodulation strategies.
With many unanswered questions, and the potential impact on more than 290 million people, the ongoing need for innovation, research and investment in the pursuit of a functional cure for HBV is clear.

