Cannabis is evolving from a stigmatized illegal drug into a socially accepted component of medicinal, wellness, and adult use products. As this metamorphosis progresses, federal agencies and informed consumers are calling for more data on both quality and safety. The industry needs to answer.
Auxly is an international cannabis company dedicated to bringing innovative, effective and high-quality cannabis products to the medical, wellness and adult-use markets. Two of the company's key wholly-owned subsidiaries are: Dosecann Inc. and KGK Science Inc., both based in Canada. Dosecann’s state-of-the-art facility is a hub for cannabis extraction, product innovation and formulation, and the company is dedicated to developing quality cannabis products that are founded in research. KGK is a contract research organization offering clinical trial services and regulatory consulting. KGK integrates scientific, clinical research, commercial and regulatory expertise to deliver well-designed solutions for propelling health and wellness, and for filling critical research gaps in the understanding of nutraceuticals, cannabis and the functional food space. Auxly’s experienced team of industry first-movers and enterprising visionaries has secured a diversified supply of raw cannabis, strong clinical, scientific and operating capabilities, and leading research and development infrastructure in order to create trusted products and brands in an expanding global market.
Cannabis grows in importance
Cannabis is a unique substance with both recreational and medicinal uses due to the presence of the well-characterized components delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Many countries have established medical marijuana programmes that provide eligible patients with access to cannabis for use under the supervision of a prescribing healthcare provider. Several European countries have decriminalized recreational use, while Uruguay, Canada, Georgia, South Africa, 10 US states, and Washington DC have legalized recreational use. Even China has entered the cannabis industry: 2 of its 34 regions are cultivating cannabis to produce CBD.
With the advent of legalization, a burgeoning industry in Canada is poised to exceed US$2 billion in annual sales over the next 2 to 3 years. Canada is expanding intended consumer uses and novel ingredient formulations for cannabis, with edibles, topicals, extracts, concentrates and synthetic cannabis constituents entering the market. Physician oversight of use is a key concern for the inclusion of cannabinoids in wellness-industry products in Canada. The appropriateness of a drug for self-use is assessed based on three principles: the need for a physician to diagnose or treat the disease; certainty about the drug effects or use; and potential for harm by the drug that can be mitigated by physician oversight. The World Health Organization’s Critical Review of CBD addressed several of the factors required to assess use in self-care, including a lack of potential for abuse1. Self-care medicinal products, encompassing cannabis-containing natural health products (NHPs) and over-the-counter (OTC) medications, are to be addressed by Health Canada in 2019 — something the agency has been reluctant to do without further research.
The US Food and Drug Administration (FDA) is in a similar position where it must deal with increased consumer demand for CBD as well as with interstate commerce allowing medical marijuana and hemp-derived CBD products. The US House of Representatives has passed report language in their version of the annual Appropriations Bill, requiring the FDA to set a safe level for CBD in foods2. A push by Congress, with similar language from the US Senate, will reduce the regulatory risk for CBD companies entering US food markets. There are already two approved prescription drugs available that include cannabis extracts: one that is pure CBD for the treatment of rare seizures in children3, and another that is a combination of CBD and THC to treat symptoms related to pain in multiple sclerosis and pain associated with cancer treatment4. Announced in the Federal Register, the FDA’s town-hall meeting with the cannabis industry on 31 May 2019 was a pivotal landmark event to seek stakeholder input toward making a decision on a future path for hemp-derived CBD food products in the US. In that Federal Register notice, FDA asked tough questions regarding hemp-derived product safety5.
Auxly and KGK provided the FDA with oral comments concerning scientific data and information about safety of products containing cannabis or cannabis-derived compounds. A more in-depth analysis followed from Auxly and KGK in their follow-up written comments to FDA on hemp-derived CBD safety6. Specifically, Auxly provided FDA with a comprehensive review on the safety of CBD in preclinical and clinical trials. Given the psychotropic properties and potential for abuse of THC, the focus of cannabis products in the wellness industry has centred around CBD. Auxly and KGK are also preparing formal comments for the Brazilian Health Regulatory Agency (Anvisa) to address the current state of scientific evidence of cannabis use for pain and other conditions treated by neurologists.
The widespread use of CBD for wellness indications is directly tied to its actions on multiple receptor systems in the body, including the endocannabinoid system, to holistically address common ailments. In Canada, the proposed use of CBD in wellness products would be in low-risk conditions, which are easily diagnosed by the public, including mitigation of stress, anxiety, poor sleep and minor pain. Although CBD has been shown to be safe at levels up to 800 mg/day in humans, potential interactions of CBD with pharmaceuticals through the p450 liver enzyme system should be investigated further. It is unknown whether these interactions with substrates, inducers, and inhibitors of cytochrome p450 2C19 and 3A4 are physiologically relevant at the serving levels proposed for wellness use. Investigation into the interaction of cannabinoids with other nutraceutical/wellness ingredients and pharmaceuticals is critical to the development of safe food and wellness products. Combinations of ingredients must be evaluated for potential pharmacological interactions, pharmacokinetic accumulation and physiological relevance that may have safety concerns. KGK Science is presently investigating the pharmacokinetics of CBD through multiple studies.
Research challenges
Determining an efficacious dose of CBD can be difficult given the paucity of data for wellness indications. This is a driving factor in the call for strict regulatory frameworks to ensure marketing claims for CBD products positioned in self-care are substantiated for the dose indicated in labelling. This will incentivize research into product-delivery technologies designed for wellness applications. In the medicinal sector, most patients opt to use cannabis through inhalation as it provides a faster and more consistent delivery of cannabinoids. Product innovation through development of effective oral or topical delivery methods may allow for easier control of therapeutic titration and mitigate hazards inherent to inhalation. Dosecann is developing a range of dosage forms focused on the effective and consistent delivery of cannabinoids. KGK will generate pharmacokinetic data to demonstrate to patients and regulatory agencies the comparable speed of onset, duration and bioavailability.
Knowledge gaps in assessing CBD use in wellness products
Regulators are looking for data in their assessment of the use of CBD as a wellness ingredient. Research in many areas must be bolstered in order to develop a robust regulatory framework.
Mode of delivery effects on safety and efficacy |
Prevalence of and exposure to CBD in food products |
Pharmacokinetics and dynamics of novel delivery modes for cannabinoids |
National level safety information about cannabinoid use and cumulative exposure data from all sources |
Long term effects of novel delivery modes for cannabinoids |
Data regarding effectiveness of policy models in legalized jurisdictions on a national and local level |
Investigation into the physiological relevance of potential drug/food interactions with cannabinoids |
Drug development with legalization of cannabinoid-containing OTC and food products |
Safety and exposure in special human populations (e.g. children, adolescents, pregnant and lactating women) |
Exposure to domestic animal populations and subsequent exposure to humans |
Degradation and genotoxicity of compounds derived from novel cannabis products |
Effectiveness of production and manufacturing quality standards for cannabis products |
Safety and efficacy data on wellness uses of cannabinoids |
Effectiveness of label warnings and instructions in self-use cannabinoid products |
Human studies which report a No Observed Adverse Effect Level (NOAEL) for use by regulatory food toxicologists |
Development of an appropriate animal model for CBD safety assessments and movement away from mouse models, which do not reflect bioavailability in humans |
Like many dietary supplement ingredients, cannabinoids present challenges in formulation as they are lipophilic compounds. Uptake of cannabinoids is notoriously difficult to optimize and has thereby spurred the industry to adopt solutions used in the dietary supplement, nutraceutical and pharmaceutical industries through creation of emulsion and micro-encapsulation strategies7. Orally consumed cannabinoids are much slower to reach maximum concentration in the bloodstream and many of the products now in development aim to overcome this hurdle. Batch consistency, a challenge for any botanically derived constituent, is addressed through control of traditional breeding practices to reduce strain variability, as well as careful timing of cultivation and processing. Companies have also moved to creating synthetic cannabinoids to eliminate unknown phytoconstituents contained in full-spectrum cannabis-derived products while also eliminating variability in the target cannabinoid arising from botanical extracts. Dosecann is applying its formulation expertise to develop products tailored to the properties of cannabinoids, doses required, purity of extract and needs of the user. Both ingestible and topical products pose difficult hurdles for regulators and the cannabis industry. Regulators must ensure the safe use of these products, protect consumers and eliminate potential harms committed through either economic adulteration of CBD ingredients or adulteration from contaminants in finished products. The cannabis industry is responsible for investigating the safety and efficacy of its products. While the side effects of THC in cannabis are well characterized8, those for CBD alone — including full spectrum CBD, CBD extracts/concentrates, synthetic CBD and other cannabis-derived cannabinoid constituents — are yet to be fully established9. The scientific gaps identified by Auxly, Dosecann and KGK, in our understanding of these cannabis products constitute the rate-limiting step (see ‘Knowledge gaps in assessing CBD use in wellness products‘). What’s more, the industry needs an appropriate model to study cannabis and cannabis-derived constituents, and it needs more preclinical and human data to determine the no-observed-adverse-effect level (NOAEL) and the margin of exposure, to determine a safe level for use in self-care products in Canada and conventional foods and dietary supplements in the US.
Avoiding the nutraceutical mistakes
When it comes to developing quality cannabis products for the wellness market, it is wise to consider the history of the nutraceutical industry, which is littered with examples of low, or no, quality control for ingredients. There are many superficial similarities, and we should learn from past mistakes. Partly, they were products of rapid growth. Starting in the late 1980s, the nutritional industry grew from small-scale, niche producers to a massive, global marketplace, driven by new ingredient innovation. For many years, regulations concerning quality of supplements and NHPs in the US and Canada struggled to keep up. It took the FDA 13 years from when it was granted Congressional authority to develop good manufacturing practices (GMPs) to releasing a GMP final rule in 200710. Industry responded, backed by a vigorous FDA inspection programme, and product quality improved.Product quality failures do still happen; however, in the Fall of 2013, the FDA encountered an outbreak of severe hepatitis linked to aegeline11, which had been rushed in as a replacement for another dietary ingredient that lacked an identity and safety dossier — 1,3-dimethylamylamine (DMAA). Manufacturers introduced aegeline without submitting a new dietary ingredient (NDI) notification and performing the identity and safety studies required by US law, with serious health consequences for consumers12.
Regulations are key to protecting people’s health and to ensuring a thriving nutritional industry. With Dosecann’s background in the pharmaceutical space, safety and quality drives decisions over product development. Auxly is setting the bar for safe, high-quality cannabis-derived products, and is taking a science-driven approach to substantiate any marketing claims.Oversight of marketing claims is one area where the nutritional industry has been historically lax. In 2012, the US Office of the Inspector General (OIG) reported an analysis of 127 dietary supplements marketed for weight loss or immune system support. The OIG reported that only 66 of 104 manufacturers submitted substantiation documents in response to their requests for information; most were not derived from human studies, and just over half qualified as background information for supplements13. Ten percent had no apparent significance in supporting any structure/function claims made about the product. The most damning conclusion was that none of the documents met all of the FDA’s recommendations for competent and reliable scientific evidence14.
CBD growth potential
To better understand the growth trajectory of the cannabis industry, one can look at the potential model of fish oil. Fish oils are grandfathered dietary ingredients in the US, with a long history of safe use in North America. They have been marketed in dietary supplements since the 1970s, but only recently has the demand for fish oil sparked innovation in new omega-3 fatty acid products and high growth forecasts. Despite their old dietary ingredient status, manufacturers and distributors have submitted multiple NDIs for fish, shellfish, and algae-derived docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) for marketing exclusivity, intellectual property protection and patent rights. The payoff in the fish oil industry has been rewarding. In a recent forecast report, the global fish oil market was expected to reach combined revenue of US$2.63 billion by 2020, but it has already hit US$3.28 billion in 2018 and is forecast to continue to US$5.42 billion by 2026. Although small by comparison, CBD products are set to overtake fish oil in the next couple of years (see ‘Comparison of omega-3 fish oil, CBD and dietary supplement markets’).
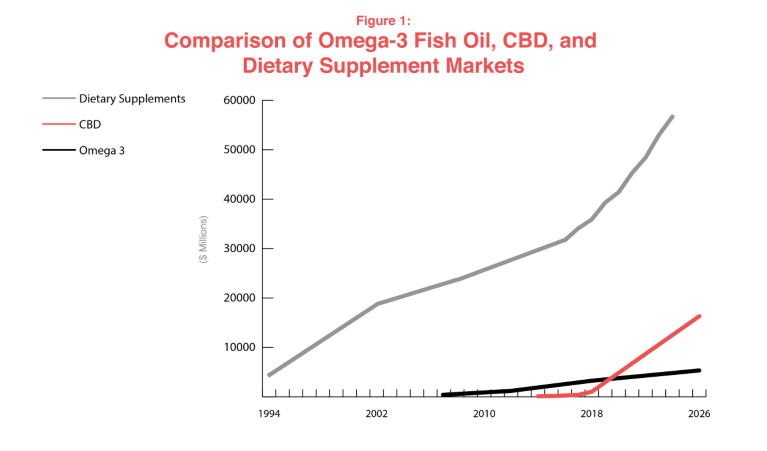
Reports and Data. (2019). Fish oil market by source, by application, by distribution channel and segment forecast. Health Food and Sports Nutrition. April 2019.
The growth in the fish oil industry comes on the back of investment in claims. Granted, not all fish oil claims have been substantiated over the years, but the structure/function claims to benefit heart health have considerable data behind them: the FDA has even accepted a qualified health claim for EPA and DHA omega 3 polyunsaturated fatty acids (PUFAs) reducing the risk of hypertension and coronary artery disease15,16. The American Heart Association recommends 2–4 g/day of EPA plus DHA from marine-derived omega 3 PUFAs for patients who need to lower their triglycerides, to be administered by a physician17,18. The safety of these omega 3 ingredients has served to maintain consumer confidence among the young generation. Millennials are more engaged with the health attributes of food when shopping19. The omega 3 industry has also created standards and shared best practices through the Global Organization for EPA and DHA Omega-320, which enabled it to expand globally and rapidly.
The omega 3 industry is a model for sustained long-term growth. It has carefully followed the NDI path to demonstrate safety, even when the relevant articles of omega 3 ingredients (EPA and DHA) were grandfathered and exempt from the notification requirement. It has also invested in clinical studies with some recognition by the FDA. Hemp or cannabis-derived cannabinoids have a similar market growth trajectory to fish oils, but do not have the same history of safe use in foods and supplements dating back to the 1970s, so cannot rely on historical use and will have to demonstrate identity and safety up front.
As well as the question of the inherent safety of CBD, there are additional potential harms in the form of fraud, economic adulteration and contaminants that need to be weeded out of the cannabis industry through tough enforcement of existing regulations, product testing and GMPs. Ingredient predecessors have come and gone over the years, and the CBD industry could yet fail if there is insufficient investment in science and in studies of efficacy, safety and quality. The industry needs to self-police to understand the number of products currently on the market that do not meet label claim. While information gaps in our understanding of CBD safety remain, it is clear that it has a safety profile more closely related to fish oil than to DMAA or aegeline.
Auxly and its partners have the determination to demonstrate maturity as leaders in providing cannabis and cannabis-derived products. The CBD market in the US already has 1,500 products — analogous to the early growth of the nutraceutical industry. How do we help the industry to demonstrate safety, quality and maturity to consumers? How will regulatory agencies move forward with enforcement to remove fly-by-night actors from the stage? Regulatory agencies like the US FDA and Health Canada must continue to do their part in the process of ensuring safety and quality in the cannabis food supply. Auxly supports strong enforcement by the regulatory authorities, including the FDA’s NDI requirement on every CBD ingredient/product sold in interstate commerce to gain better control of ingredient identity and safety and to protect investments by responsible companies. GMP facility inspections by regulatory agencies worldwide will play a critical role in ensuring product quality in the cannabis marketplace. Vigorous compliance with regulations by industry and consistent enforcement by healthcare agencies will be paramount to the future success of the cannabis industry. It does not take many enforcement examples to scare the rest of the industry onto the straight and narrow path of regulatory compliance.
Looking into the future
In his 1948 speech to the House of Commons, Winston Churchill stated that those who fail to learn from history are condemned to repeat it. Will manufacturers and retailers of CBD conventional foods, dietary supplements, edibles and self-care products (cosmetics and NHPs) learn from past mistakes of the dietary supplement industry or will they write a different narrative? The cannabis industry has captured the attention of manufacturers, retailers, trade-show event organizers and consumers. Auxly’s plan is to ensure this industry stays around for a long, healthy future, backed by rigorous, scientific data.
Auxly’s approach is methodical with the thought that manufacturers and retailers of cannabis and cannabis-derived constituents, including CBD, have the chance to experience unprecedented market growth, maintain consumer confidence, and offer innovative global products for decades to come. Auxly and its partners recognize the need to invest in the science, substantiation/efficacy and safety of the cannabis industry today to set the bar high for products entering the market place. It will take time and considerable financial investment in science and manufacturing quality to write the future narrative of the CBD industry and chart a mature course. The industry is undergoing a scientific renaissance, and Auxly is at the forefront providing a careful plan to innovate the marketplace. Our advantage is that Auxly, Dosecann, and KGK can learn from the mistakes of the past by investing now for our future.
Auxly and its partners are working to effect responsible change and ensure maturation in the cannabis industry. While preclinical studies show a wide therapeutic and health wellness potential for cannabis and cannabis constituents, Auxly is working to bring clarity to key scientific questions surrounding safety, pharmacokinetics, and claims. The group is currently working to bring safe products to market with claims backed by competent and reliable scientific information.
Contact Auxly today to learn more and discuss product development needs. Contact KGK Science for interest in pursuing cannabis or cannabinoid research.

