BSIP SA / Alamy Stock Photo
KAIMRC researchers have unveiled how the so-called transmembrane receptor CD-95 governs the programmed death, or apoptosis, of lymphoma cells. This breakthrough is expected to give rise to novel therapies against aggressive forms of cancer, such as Burkitt’s lymphoma (BL).
More prevalent in Africa than in the United States and Western Europe, BL is a rare type of lymphoma that affects adults and children. It starts in immune system cells called B-cells before growing and spreading rapidly in the body. Existing anti-cancer therapies, including chemotherapy and radiation, have proven unsuitable against BL because of its aggressiveness. Specifically, when applied to BL, these conventional approaches are linked to high risk of tumour lysis syndrome, a potentially fatal complication that disrupts electrolyte and metabolite levels in blood resulting from the release of cancer cell content into the bloodstream.
Sabine Matou-Nasri and co-workers investigated the molecular mechanisms underlying CD95-mediated apoptosis to eliminate these side-effects and improve the clinical outcomes of anti-BL treatments. “Elucidating these mechanisms was necessary to identify novel therapeutic targets and enhance the efficacy and specificity of immunotherapy,” she adds.
The researchers evaluated the response of lymphoma B-cells to anti-CD95 monoclonal antibody, which specifically binds to and activates the transmembrane receptor expressed in these malignant cells.
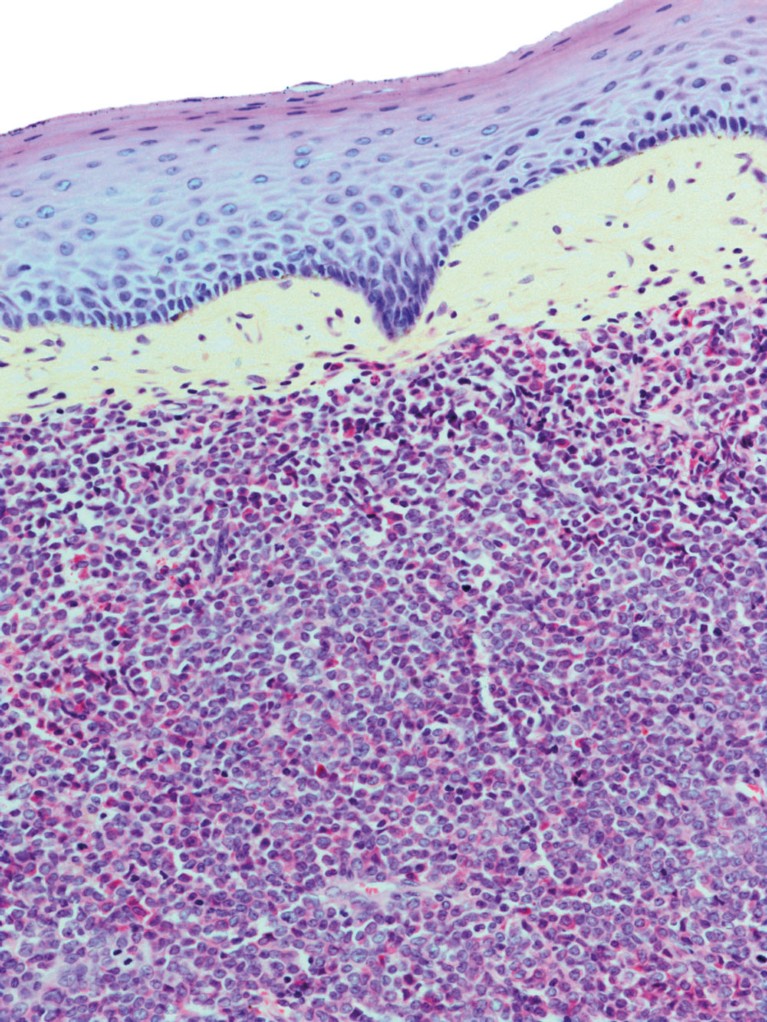
Burkitt’s lymphoma is a rare form of cancer that is more common in Africa than in the US and Western Europe.STEVE GSCHMEISSNER /SPL / Getty Images
The antibody treatment caused the cancer cells to undergo apoptosis. Furthermore, the expression of the Pim-1 protein, which stimulates cell survival and proliferation in addition to inhibiting apoptosis, decreased. This antibody treatment also reduced the cell viability and phosphorylation of protein kinase B, an enzyme involved in cell survival pathways, by 50% in 72 hours. However, the team found that the cell death rate remained below 46%.
According to Matou-Nasri, her team has demonstrated that the anti-CD95 monoclonal antibody treatment partly destroyed BL because of the partial inhibition of survival pathways. “Our results have proven that targeting Pim-1 is insufficient to fully eradicate BL, which suggests a need for combinatory treatments against other main survival cytoplasmic proteins,” she adds, noting the current tremendous efforts deployed to develop pharmacological inhibitors that block Pim-1 anti-apoptotic activities. These treatments would associate the anti-CD95 monoclonal antibody with target-specific chemotherapeutic agents.
“In future work, we are planning to undertake a gene–immune therapy approach against BL by knocking down the gene expressing Pim-1, then targeting its induced cell surface proteins that are likely to be involved in cell survival,” says Matou-Nasri.


