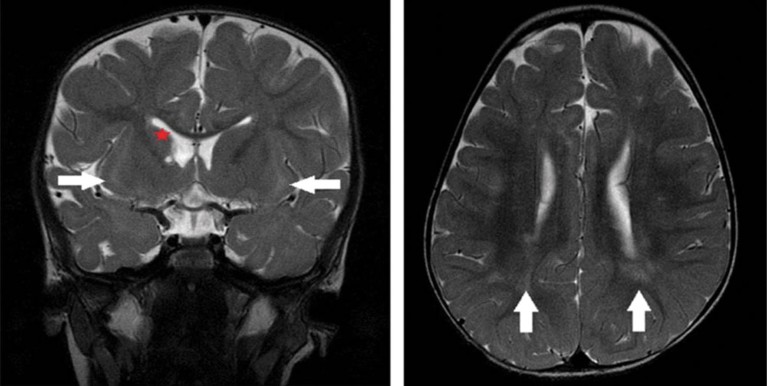
An MRI image of the brain of the Saudi girl, showing hyper-intensity in the white matter, caused by the accumulation of glycine.© ©MAJID ALFADHEL / HUMAN GENETICS
The rare genetic disorder known as glycine encephalopathy is usually caused by defects in the enzyme system that breaks down the amino acid glycine, resulting in an accumulation of the protein building block in the body’s tissues and fluids. Now, a team, led by geneticists from KAIMRC and King Saud bin Abdulaziz University for Health Sciences, has discovered that mutations in a protein that controls the shuttling of glycine within the brain can also trigger the disease.
In a case report, Majid Alfadhel and his colleagues describe an infant girl, born to Saudi first cousins, who had all the clinical symptoms of glycine encephalopathy; which is also known as non-ketotic hyperglycinemia and affects an estimated one in 60,000 newborns worldwide.
Her symptoms included breathing problems, seizures, low muscle tone and elevated glycine levels in various bodily fluids. Yet, the patient harboured no disease-causing mutations in any of the three glycine degradation genes previously implicated in the disorder.
In search of a genetic culprit, Alfadhel’s team sequenced all the protein-coding portions of the infant’s genome. They honed in on a defect in the SLC6A9 gene that encodes the glycine transporter 1 (GlyT1) protein.
The patient had two copies of this defective mutation, while her parents and older sister all had just one copy, indicating that the aberrant DNA acts in a recessive fashion. Structural analysis showed that the mutation alters the shape and chemical properties of the GlyT1 protein to disrupt its normal function. Previous research revealed that the mutation causes a disease in rodents that’s similar to the one in human patients.
When Alfadhel told the parents of the affected child that he had discovered the molecular offender responsible for their daughter’s disorder, “they were very happy,” he says, “because they had struggled for a long time, going from clinic to clinic without a diagnosis.” Now, that family — and others around the world — can undergo pre-implantation genetic testing to avoid passing on the disease to future offspring.
Although the clinical consequences of this newly discovered mutation are the same as other known genetic defects responsible for glycine encephalopathy, the mechanism is quite different. GlyT1 mutations only lead to glycine build-up in the brain, whereas defects in glycine degradation cause a backlog of the amino acid throughout the body. The importance of this distinction is unclear, however, and further cases are needed to “confirm the full spectrum of SLC6A9-related phenotypes and genotypes,” says Alfadhel.


