Groundbreaking research on the differentiation of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) has shown that stem cells can be differentiated into retinal cells and three-dimensional (3D) retinal organoids with all the major retinal cell types. These technological advances offer an unparalleled opportunity to model human retinal development and disease, test potential therapeutic drugs and develop cell transplantation treatments (Fig. 1).
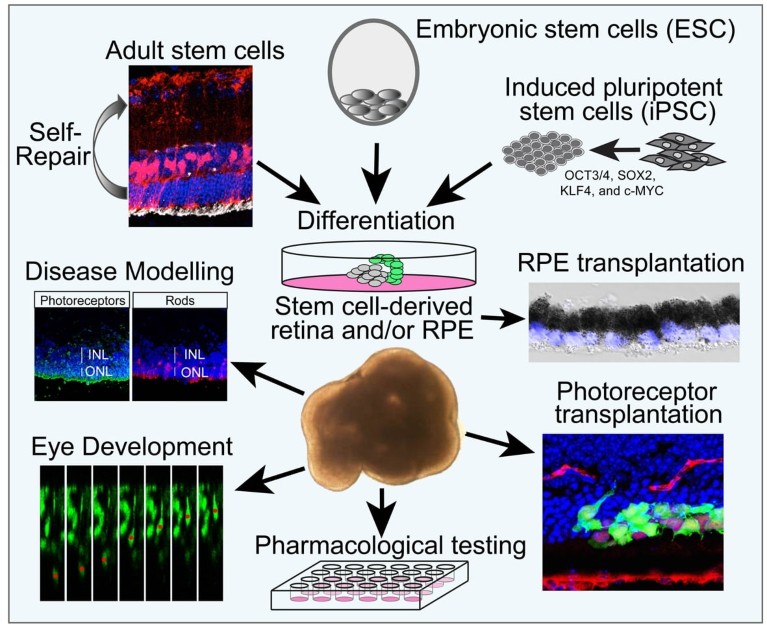
Figure 1: Schematic showing how endogenous retinal stem cells, embryonic stem cells and induced pluripotent stem cells can be differentiated into retinal cells with a wide range of applications.
The development of patient iPSC-derived models allows us to interrogate disease mechanisms for inherited retinal dystrophies, and test therapies in genomic and cellular context.
We used patient iPSC-derived retinal pigment epithelium (RPE) and retinal organoids for a common RP2 nonsense mutation that causes X-linked retinitis pigmentosa, to test if translational read-through-inducing drugs (TRIDs) could restore RP2 protein expression. In iPSC-derived RPE and retinal organoids, TRIDs restored sufficient RP2 protein to reverse the observed cellular phenotypes, indicating that TRIDs could have clinical application for specific nonsense mutations in retinal dystrophy genes1.
CEP290 mutations cause syndromic ciliopathies, but can also cause Leber congenital amaurosis (LCA). A deep intronic mutation is the predominant cause of LCA, which stimulates insertion of a cryptic exon resulting in premature termination. LCA patient-derived iPSC models revealed that the highest levels of aberrant transcript were detected in retinal organoids, co-incident with photoreceptor differentiation and the splicing of photoreceptor-specific exons, suggesting tissue-specific differences in splicing are likely to contribute to retinal expression of disease2. Treatment with an antisense oligonucleotide (AON) supressed cryptic exon inclusion and improved normal splicing. CEP290 protein expression was suffi cient to rescue ciliation in retinal organoids2. These data demonstrated that an AON therapy could be a practical therapeutic option for treating CEP290-LCA, and an AON therapy is now in phase I/II clinical trials for CEP290-LCA.
Cell replacement therapy offers a therapeutic approach for patients with advanced disease in which there has been loss of photoreceptors, RPE or retinal ganglion cells (RGCs). Age-related macular degeneration (AMD) is a major cause of blindness, leading to loss of the RPE and central vision. We developed a human ESC-derived monolayer of RPE on a supportive synthetic membrane as a ‘patch’ for transplantation to replace lost RPE cells. A phase I clinical trial was designed to test the safety and feasibility of implantation of a patch in two subjects with acute wet AMD3. At 12 months post-transplantation there was evidence of retinal function over the patch and a gain in visual acuity in the two patients3. This study provides an early indication of the safety and feasibility of manufacturing ESC-RPE patches as a potential treatment for AMD.
In advanced AMD, as well as in inherited disorders, the photoreceptors are lost. We have previously shown that transplantation of photoreceptor precursors derived from donor tissue can restore visual function in a mouse model of congenital stationary night blindness4. With further development of 3D differentiation protocols, we are now able to achieve similar results with photoreceptor precursors generated from both mouse and human ESC-derived retinal organoids, and are applying these techniques to treat advanced degeneration5.
In lower vertebrates, Müller glial cells in the retina have stem cell properties and can regenerate lost neurons. We are investigating how these cells might be utilised in the human retina to induce endogenous repair, as a source of neuroprotective factors or to generate new RGCs for transplantation in the treatment of glaucoma. These complementary transplantation studies demonstrate the utility of stem cells, and feasibility of transplantation as a therapeutic strategy for restoring vision when the retina has degenerated. The studies are particularly important when we consider that stem cells may also become a source of gene-edited cells for transplantation.



 Nature Outline: Retinal repair
Nature Outline: Retinal repair