Credit: AIP Emilio Segrè Visual Archives, Physics Today Collection
Donald Caspar defined the rules that govern the self-assembly of simple viruses. This laid the foundations for a new way of thinking about the molecular systems that regulate and drive all living cells. These rules made it straightforward to characterize other viruses, and then to design strategies to combat them. The same rules are also essential in designing viral vectors to deliver gene therapy.
Viruses typically consist of a strand of genetic material — DNA or RNA — enclosed in a coat of protein molecules. Using the laws of thermodynamics and the constraints of symmetry, Caspar catalogued the ways in which proteins can come together to form the icosahedral shells of spherical viruses (these include rhinovirus and poliovirus) and the helical lattices of rod-shaped viruses (such as Ebola).
How the coronavirus infects cells — and why Delta is so dangerous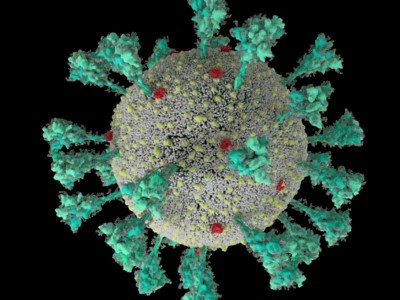
In the mid-twentieth century, few protein structures had been solved at atomic resolution. Caspar used limited data from X-ray crystallography, electron microscopy and fibre diffraction to tease out features of prototypical viruses. With an artist’s eye, unbounded curiosity and a passion for ‘connecting the dots’, he created exquisite sketches of virus structures and membranes, which still grace the pages of many texts.
Caspar was born in 1927 in Ithaca, New York, when his father was a graduate student in chemistry at Cornell University. When he was ten years old, a family friend, the crystallographer Isidor Fankuchen, told him of the recent discovery that particles of the rod-shaped tobacco mosaic virus (TMV) had a semi-crystalline structure, suggesting that the virus comprised many identical units. This set him on a lifelong journey to understand its structure and behaviour. At the Polytechnic Institute of Brooklyn in New York City, he was the youngest student on a two-week course on X-ray crystallography run by Fankuchen. After graduating in physics at Cornell, Caspar did his PhD on the structure of TMV at Yale University in New Haven, Connecticut. He then held postdoc positions at the California Institute of Technology in Pasadena, and at the MRC Laboratory of Molecular Biology in Cambridge, UK.
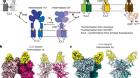
At Cambridge in 1956, he set out to prove the theory advanced by James Watson and Francis Crick that spherical virus particles had cubic symmetry (in other words, that the protein coat was made of identical subunits arranged in equivalent ways). He demonstrated that tomato bushy stunt virus had icosahedral symmetry. It soon became clear that other viruses were also icosahedral. This, the most complex of the cubic symmetries, requires 60 identical subunits. But biochemical data indicated that many virus particles had a lot more.
Caspar resolved this paradox in the late 1950s, working with Aaron Klug at Birkbeck College in London, and taking a clue from architect Buckminster Fuller’s geodesic domes. He introduced quasi-equivalence — the idea that equivalent protein subunits could vary slightly in conformation to tightly seal the viral shells. This opened up a universe of possibilities for the controlled assembly and disassembly of everything inside a living cell. Twenty years later, Caspar’s own lab demonstrated that viral proteins can switch between non-equivalent conformations where necessary.
Caspar’s PhD work on TMV was the basis for a paper published back-to-back with one by Rosalind Franklin in 1956 (D. L. D. Caspar Nature 177, 928 (1956); R. E. Franklin Nature 177, 928–930; 1956). These pinpointed the viral RNA deep within the helical protein rods and marked the start of a friendship that lasted until Franklin’s death two years later. In 2008, their relationship was dramatized in Anna Ziegler’s play Photograph 51, with romantic overtones that Don insisted were fictional.
In 1958, Caspar established what would become one of the most influential centres for the study of macromolecular structure: the Laboratory for Structural Biology at the Children’s Cancer Research Foundation at Boston Children’s Hospital. The lab was moved to Brandeis University in Waltham, Massachusetts, in 1972. In 1994, Caspar moved to the Institute for Molecular Biophysics at Florida State University in Tallahassee, where he spent the remainder of his career.
Writing was a struggle for Don. Editing a manuscript often devolved into an infinite loop. He wanted to say everything first — every statement was somehow a prerequisite for every other. He was much better at talking. In fact, to those of us who collaborated with Don, it was never obvious when he actually got any work done. He never stopped talking. He could eat his entire lunch without missing a beat. Group members would set a lab timer before going to ask him a question. When the timer went off, they could plead the need to check an experiment.
Don’s many passions included gardening and Grecian urns. In a walk around his garden in Cataumet, on Cape Cod in Massachusetts, he would relate the family, genus and common name of every plant and tree and expound on why he chose a particular variety and colour for each location. Go with him to a museum, and it was never time to leave without a visit to the urns. He was married for 50 years to Gwladys Caspar, an early biosafety officer at Harvard University in Cambridge, Massachusetts. Her quick guide to biosafety levels 1–4 (don’t eat it, don’t touch it, don’t breathe it and don’t do it here) is still taught.
Don was brilliant, kind and always enthusiastic about new ideas — his own and others’. His support for young scientists was extraordinary, as was his advocacy for women in science. Don’s deep insight and broad perspective will be sorely missed.


 Aaron Klug (1926–2018)
Aaron Klug (1926–2018)
 How the coronavirus infects cells — and why Delta is so dangerous
How the coronavirus infects cells — and why Delta is so dangerous