Spinal-cord stimulation has helped three paralysed people to walk.Credit: Jean-Baptiste Mignardot
Not so long ago, the hope that someone paralysed for years by a severe spinal-cord injury would ever be able to walk again was just that — hope. But recent advances are bringing those hopes closer to reality.
In this week’s Nature, researchers describe a treatment — a combination of electrical stimulation of the spinal cord and physical therapy — that has enabled three men with spinal-cord injury to walk (F. B. Wagner et al. Nature 563, 65–71; 2018). And this is not just in controlled laboratory conditions: they have been able to take walks outside again.
Read the paper: Targeted neurotechnology restores walking in humans with spinal cord injury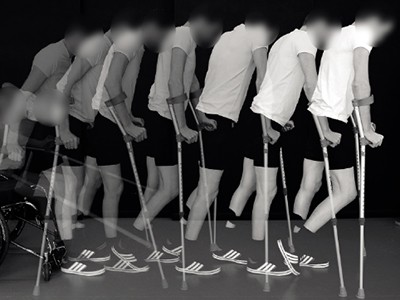
It’s an extraordinary development that could have implications for hundreds of thousands of people around the world. And it’s also the result of decades of cross-disciplinary research that has steadily built an evidence base in animal experiments — with the scientists involved sometimes facing criticism for doing them — and taken that work carefully into the clinic.
Researchers have long pursued diverse strategies to repair and reactivate the spinal cord after injury. Many approaches are remarkably effective in regenerating and achieving functional recovery in mice and other animals, but fail to translate to human therapies. The advance in the current study was that, rather than delivering a constant electric current — as had been tried before — the researchers applied patterns of stimulation calculated to activate the correct groups of leg muscles at the correct time during stepping. In this way, specific locations in the spinal cord could be targeted, to activate the muscles in a coordinated fashion. This patterned stimulation protocol not only allowed the unprecedented restoration of walking ability, but also enabled the individuals to regain control over previously paralysed muscles when electrical stimulation was turned off. This indicates that the brain and spinal cord had re-established functional connections, revealing an unexpected degree of plasticity.
In light of such progress, the prognosis for what was long considered an irreversible condition seems a lot brighter. But there is much more work to do. Spinal injuries vary enormously in their location, severity and outcome, and it will take many more studies to understand who will benefit from this technology. The current research is a proof of concept in a small number of participants who had a range of residual leg function at the start of the study. A major challenge is to understand what determines successful recovery. For example, one source of variability might be how much sensory information the damaged spinal cord can still transmit to the brain.
In a related study published this week in Nature Neuroscience, the same team shows that continuous stimulation (which is enough to restore locomotion in rodents) is less effective in humans because it interferes with the transmission to the brain of sensory feedback about an individual’s own movements and body position (E. Formento et al. Nature Neurosci. https://doi.org/10.1038/s41593-018-0262-6; 2018). This is another reason why temporally patterned stimulation could be more effective, and might have been one key to success for the three participants in the Nature study. However, different stimulation methods might turn out to be more or less useful for different individuals.
It’s also important to temper this exciting success story with caution about access. According to the World Health Organization, between 250,000 and 500,000 people around the globe are affected by a spinal-cord injury each year — most caused by road accidents, falls or violence. Spinal stimulation is a complex and expensive medical procedure, and recovery also seems to require intensive rehabilitation. It will not be available to all — at least, any time soon. But it is a first step.

 Watch: Spinal injury patients walk again
Watch: Spinal injury patients walk again
 Three people with spinal-cord injuries regain control of their leg muscles
Three people with spinal-cord injuries regain control of their leg muscles
 Read the paper: Targeted neurotechnology restores walking in humans with spinal cord injury
Read the paper: Targeted neurotechnology restores walking in humans with spinal cord injury
 Reducing neuronal inhibition restores locomotion in paralysed mice
Reducing neuronal inhibition restores locomotion in paralysed mice
 Repairing the neural highway
Repairing the neural highway