- NEWS AND VIEWS
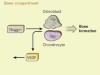
Receptor becomes a ligand to control bone remodelling
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 561, 180-181 (2018)
doi: https://doi.org/10.1038/d41586-018-05960-x
References
Zaidi, M. Nature Med. 13, 791–801 (2007).
Nakashima, T. et al. Nature Med. 17, 1231–1234 (2011).
Xiong, J. et al. Nature Med. 17, 1235–1241 (2011).
Ikebuchi, Y. et al. Nature 561, 195–200 (2018).
Huynh, N. et al. J. Dent. Res. 95, 673–679 (2016).
Singha, U. K. et al. J. Cell Biochem. 103, 434–446 (2008).
Zaidi, M., Yuen, T., Sun, L. & Rosen, C. J. Endocr. Rev. https://doi.org/10.1210/er.2018-00050 (2018).
Anderson, D. M. et al. Nature 390, 175–179 (1997).
Shimamura, M. et al. Proc. Natl Acad. Sci. USA 111, 8191–8196 (2014).
Hanada, R. et al. Nature 462, 505–509 (2009).

 Read the paper: Coupling of bone resorption and formation by RANKL reverse signalling
Read the paper: Coupling of bone resorption and formation by RANKL reverse signalling
 Vessels of rejuvenation
Vessels of rejuvenation
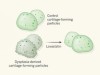 Statins give bone growth a boost
Statins give bone growth a boost