- NEWS AND VIEWS
A lymphatic waste-disposal system implicated in Alzheimer’s disease
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 560, 172-174 (2018)
doi: https://doi.org/10.1038/d41586-018-05763-0
References
Alitalo, K., Tammela, T. & Petrova, T. V. Nature 438, 946–953 (2005).
Mascagni, P. Vasorum lymphaticorum corporis humani historia et ichnographia (Pazzini Carli, 1787).
Aspelund, A. et al. J. Exp. Med. 212, 991–999 (2015).
Louveau, A. et al. Nature 523, 337–341 (2015).
Absinta, M. et al. eLife 6, e29738 (2017).
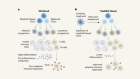
Da Mesquita, S. et al. Nature 560, 185–191 (2018).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Nature Rev. Neurol. 14, 133–150 (2018).
Zhao, Z. et al. Nature Neurosci. 18, 978–987 (2015).
Xie, L. et al. Science 342, 373–377 (2013).
Courtice, F. C. & Simmonds, W. J. Aust. J. Exp. Biol. Med. Sci. 29, 255–263 (1951).
Shibata, M. et al. J. Clin. Invest. 106, 1489–1499 (2000).
Deane, R. et al. Neuron 43, 333–344 (2004).

 Read the paper: Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease
Read the paper: Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease
 Immune memory in the brain
Immune memory in the brain
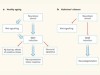 A protective factor for the ageing brain
A protective factor for the ageing brain