Abstract
Fbw7 and Cdh1 are substrate-recognition subunits of the SCF- and APC-type E3 ubiquitin ligases, respectively. There is emerging evidence suggesting that both Fbw7 and Cdh1 function as tumor suppressors by targeting oncoproteins for destruction. Loss of Fbw7, but not Cdh1, is frequently observed in various human tumors. However, it remains largely unknown how Fbw7 mechanistically functions as a tumor suppressor and whether there is a signaling crosstalk between Fbw7 and Cdh1. Here, we report that Fbw7-deficient cells not only display elevated expression levels of SCFFbw7 substrates, including cyclin E, but also have increased expression of various APCCdh1 substrates. We further defined cyclin E as the critical signaling link by which Fbw7 governs APCCdh1 activity, as depletion of cyclin E in Fbw7-deficient cells results in decreased expression of APCCdh1 substrates to levels comparable to those in wild-type (WT) cells. Conversely, ectopic expression of cyclin E recapitulates the aberrant APCCdh1 substrate expression observed in Fbw7-deficient cells. More importantly, 4A-Cdh1 that is resistant to Cdk2/cyclin E-mediated phosphorylation, but not WT-Cdh1, reversed the elevated expression of various APCCdh1 substrates in Fbw7-deficient cells. Overexpression of 4A-Cdh1 also resulted in retarded cell growth and decreased anchorage-independent colony formation. Altogether, we have identified a novel regulatory mechanism by which Fbw7 governs Cdh1 activity in a cyclin E-dependent manner. As a result, loss of Fbw7 can lead to aberrant increase in the expression of both SCFFbw7 and APCCdh1 substrates. Our study provides a better understanding of the tumor suppressor function of Fbw7, and suggests that Cdk2/cyclin E inhibitors could serve as effective therapeutic agents for treating Fbw7-deficient tumors.
Similar content being viewed by others
Introduction
Deregulation of the cell cycle can lead to uncontrolled proliferation, defects in chromosome segregation and ultimately cancer development1,2,3. Therefore, proteins required for proper cell cycle progression must be tightly regulated and perform their functions only when needed. Ubiquitin ligases are key components of this control as they ubiquitinate key regulators of the cell cycle machinery, signaling for their destruction4. Two major classes of E3 ligases, SKP1-CUL1-F-box protein (SCF)- and Anaphase Promoting Complex/Cyclosome (APC/C)-type ubiquitin ligases, are known to target multiple substrates crucial for cell cycle progression5,6. SCF complexes require F-box proteins for target identification, while APC/C utilizes activators, such as Cdh1 and Cdc20, for substrate recognition4. These two families of proteins orchestrate the temporal expression of cell cycle controllers and ensure restricted cell proliferation and faithful DNA segregation.
One well-studied F-box protein is F-box and WD repeat domain-containing 7 (Fbw7). The first member of this gene family was originally identified in budding yeast, and was named Cdc47. Human Fbw7 is located on chromosome 4 and encodes three transcripts (isoforms α, β and γ)8. These three isoforms vary at the N-terminal region, but contain conserved interaction domains in the C terminus (F-box and WD40 repeats). The F-box motif of each F-box protein is a region of approximately 40 amino acids that recruits other subunits of the SCF complex by interacting with S-phase kinase-associated protein 1 (Skp1)9, thus allowing the formation of a functional E3 ligase complex5,10. Also, the C-terminal region of Fbw7 contains a stretch of eight WD40 repeats that bind phosphorylated substrates11,12. Characterized substrates of Fbw7 include cyclin E, c-Myc, c-Jun, and NOTCH-1, all of which are well-known products of oncogenes involved in a variety of human tumors6,7,13. Not surprisingly, Fbw7 is a tumor suppressor whose mutations occur in multiple neoplasms including breast cancer, colon cancer and leukemia14,15,16,17,18. Approximately 6% of all primary human tumors harbor mutations in the Fbw7 gene with the highest mutation rates found in cholangiocarcinoma and T-cell acute lymphoblastic leukemias (T-ALL)7. The roles of SCFFbw7 substrates in tumorigenesis have been well documented. However, how Fbw7 suppresses tumor formation is currently not well understood. Although negative regulation of c-Myc and NOTCH-1 by Fbw7 has been implicated in tumorigenesis, their contributions to the tumor suppressor function of Fbw7 still require substantial characterization16,18,19,20. On the other hand, cyclin E has garnered much attention as a possible key mediator for the ability of Fbw7 to inhibit tumorigenesis. To that end, Lengauer and colleagues21 demonstrated that loss of Fbw7 function led to genomic instability that could be suppressed by the additional depletion of cyclin E. Furthermore, a cyclin E mutant (T380A) that cannot be degraded by Fbw7 induced genomic instability more effectively than wild-type (WT) cyclin E22. Moreover, mice with the murine equivalent of the T380A mutation exhibited chromosomal instability and increased cancer development23. Although emerging evidence suggests that controlling Cdk2/cyclin E kinase activity likely accounts for the tumor suppressor function of Fbw7, considerable amount of work is still needed to elucidate the exact role of cyclin E in tumorigenesis. The best-characterized function of cyclins is to bind and activate cyclin-dependent kinases (CDKs), leading to phosphorylation of downstream substrates24. Thus, it is critical to pinpoint the major downstream targets of cyclin E, which may mediate the tumor suppressor function of Fbw7. Interestingly, Cdk2/cyclin E was reported to inactivate APCCdh1, through direct phosphorylation of its activator, Cdh125.
APC/C is a well-characterized E3 ligase important for genomic stability and a known tumor suppressor26. Cdh1 is an activator and a substrate-recognition subunit of the APC/C E3 ligase. Unlike F-box proteins that bind phosphorylated substrates, activators of APC/C recognize Destruction Box (D-Box) or KEN Box motifs27,28. Substrates of APCCdh1 include proteins important for G1 stability (cyclin A, cyclin B1 and Skp2), DNA synthesis (Cdc6 and Geminin) and mitosis (Aurora A and Plk)29,30,31,32. Therefore, APCCdh1 is a critical regulator of cell cycle progression. As aforementioned, aberrant cell cycle regulation can often lead to missegregation of chromosomes, resulting in aneuploidy. Not surprisingly, many reports have implicated Cdh1 function in maintaining proper genomic stability26,33,34,35. In addition, multiple APCCdh1 targets have also been documented for their oncogenic capabilities. Elevated levels of Skp2 can promote the development of prostate carcinomas36 and facilitate the transformation of Rat1 cells37. Both Plk1 and Aurora A are also amplified in various tumors and their expression correlates with poor clinical prognosis38,39. Furthermore, transgenic mice overexpressing cyclin A develop acute myeloid leukemia40. Although Cdh1 displays many characteristics of a bona fide tumor suppressor, only recently has this been established. Garcia-Higuera et al.26 demonstrated that heterozygous Cdh1 mice (Cdh1+/−) develop epithelial tumors, suggesting a haploinsufficient role of Cdh1 in tumor suppression. Moreover, reduced Cdh1 expression was observed in hematological neoplasias and solid tumors33. As loss of Cdh1 function can have multiple effects that predispose cells to transformation, it is critical to understand its regulatory pathways.
Our present study has identified a novel signaling link between the Fbw7 and Cdh1 tumor suppressor pathways. Fbw7 governs the E3 ligase activity of APCCdh1 by modulating the expression of cyclin E. Thus, in Fbw7-deficient cells, elevated cyclin E expression leads to the inactivation of APCCdh1 via direct phosphorylation of Cdh1. This subsequently results in elevation of multiple known APCCdh1 substrates, many of which are well-characterized oncoproteins. These findings highlight a critical role of Fbw7 in suppressing tumorigenesis. Loss of Fbw7, which is frequently observed in various human cancers, can lead to aberrant inactivation of both the Fbw7 and Cdh1 signaling pathways, thus providing a mechanistic insight into the tumor suppressive function of Fbw7.
Results
Depletion of Fbw7 leads to aberrant elevation of APCCdh1 substrates
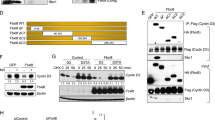
Loss of Fbw7 activity is frequently observed in many types of human cancers, with the highest mutation rates found in T-ALL (approximately 30%)7. Interestingly, we noticed that in Fbw7-deficient T-ALL cell lines, besides the SCFFbw7 substrate cyclin E, the expression of APCCdh1 substrates cyclin B and cyclin A was also elevated (Figure 1A). However, the underlying mechanism accounting for this upregulation is currently unclear. Similarly, elevated expression of other APCCdh1 substrates such as Plk1 and Cdc20 was also observed in Fbw7-deficient breast cancer cell lines (Supplementary information, Figure S1A). These results indicate a possible crosstalk between the Fbw7 and Cdh1 tumor suppressor pathways. We further demonstrated that compared with WT DLD1 colon cancer cells, loss of Fbw7 led to a significant induction of various APCCdh1 substrates including cyclin A, cyclin B, Plk1, Cdc20 and Skp2 (Figure 1B and Supplementary information, Figure S1B). Moreover, we observed a significant decreased expression of various Skp2 substrates including p130, p57 and p27, consistent with the model that loss of Fbw7 leads to reduced Cdh1 activity, which subsequently causes elevated Skp2 activity (Figure 1B).
Depletion of Fbw7 leads to elevated expression of APCCdh1 substrates. (A) Immunoblot analysis of whole-cell lysates (WCL) derived from various T-ALL cell lines with the indicated antibodies. Where indicated, the Fbw7 genetic status is labeled for the various T-ALL cell lines. (B) Immunoblot analysis of WT or Fbw7−/− DLD1 colon cancer cells synchronized by growth in nocodazole, and then released for the indicated periods of time. (C-D) FACS analysis of WT (C) or Fbw7−/− (D) DLD1 cells synchronized by growth in nocodazole, and then released for the indicated periods of time. (E) Immunoblot analysis of WCL derived from HEK 293 cells infected with the indicated shRNA lentiviral vectors followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells. (F) Immunoblot analysis of WCL derived from Fbw7−/− DLD1 cells infected with a retroviral construct encoding HA-Fbw7 (with empty vector (EV) as a negative control) followed by selection in 1 μg/ml puromycin for 96 h to eliminate the non-infected cells.
Furthermore, FACS analysis indicated that depletion of Fbw7 did not cause a significant change in cell cycle progression (Figure 1C and 1D), suggesting that the change in APCCdh1 substrates was not a secondary effect derived from gross misregulation of the cell cycle. Furthermore, we did not observe a significant change in Cdh1 expression after loss of Fbw7, suggesting that Fbw7 does not regulate APCCdh1 substrates by modulating the abundance of Cdh1 itself (Figure 1B and 1E). Consistently, shRNA-mediated depletion of Fbw7α and Fbw7γ, and to a lesser extent, Fbw7β, led to a similar upregulation of APCCdh1 substrates as that observed in Fbw7−/− cells (Figure 1E and Supplementary information, Figure S1C). Furthermore, reintroduction of WT-Fbw7 led to a dramatic decrease in the expression of various Cdh1 substrates in the Fbw7−/− DLD1 cells (Figure 1F), suggesting a causal relationship between the loss of Fbw7 function and the aberrant upregulation of APCCdh1 substrates.
Cyclin E plays a central role in mediating the ability of Fbw7 to regulate APCCdh1 substrate abundance
Although multiple SCFFbw7 substrates have been identified, it is still unclear how they contribute to the tumor suppressor function of Fbw7. A recent study21 has highlighted a possible role of cyclin E in mediating the ability of Fbw7 to suppress tumorigenesis, as depletion of cyclin E rescued the genomic instability observed in Fbw7−/− cells. Additionally, cyclin E has been implicated in regulating Cdh1 activity25. These clues prompted us to further investigate whether cyclin E is the major route through which Fbw7 modulates APCCdh1 substrate abundance. Indeed, we found that depletion of cyclin E via multiple independent shRNA lentiviral vectors led to marked decreases in the expression of the APCCdh1 substrates cyclin A and Plk1 in asynchronized Fbw7−/− DLD1 cells (Figure 2A). More importantly, depletion of cyclin E in Fbw7−/− cells rescued the aberrant expression of APCCdh1 substrates to levels comparable to those in WT DLD1 cells in synchronized conditions (Figure 2B). Furthermore, the reintroduction of an shRNA-resistant cyclin E-encoding construct in cyclin E-depleted Fbw7−/− cells reinstated the aberrant APCCdh1 substrate expression, further suggesting that cyclin E is a critical intermediate signaling component through which Fbw7 regulates APCCdh1 E3 ligase activity (Figure 2C).
Cyclin E is a critical component in mediating the ability of Fbw7 to regulate APCCdh1 substrate abundance. (A) Immunoblot analysis of WCL derived from Fbw7−/− DLD1 cells infected with the indicated shRNA lentiviral vectors followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells. (B) Immunoblot analysis of WT or Fbw7−/− DLD1 cells synchronized by growth in nocodazole, and then released for the indicated periods of time. Where indicated, Fbw7−/− DLD1 cells were infected with an shcyclin E lentiviral vector (with an shGFP vector as a negative control) followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells before the synchronization. (C) Immunoblot analysis of WCL derived from Fbw7−/− DLD1 cells stably infected with the indicated shRNA lentiviral vectors followed by transfection with a vector encoding an shRNA-resistant cyclin E26 or an empty vector as a negative control. (D) Immunoblot analysis of Fbw7−/− or WT DLD1 cells synchronized by growth in nocodazole, and then released for the indicated periods of time. Where indicated, WT DLD1 cells were infected with a retrovirus vector encoding WT cyclin E or a non-degradable version of cyclin E (T380A) (with the empty vector as a negative control) followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells before the synchronization. (E) Immunoblot analysis of WCL derived from Fbw7−/− DLD1 cells treated with 20 μM Roscovitine for 6 h before harvesting. (F) Immunoblot analysis of WCL derived from Fbw7−/− DLD1 cells infected with the indicated shRNA lentiviral vectors followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells. (G) Immunoblot analysis of WCL and anti-Cdc27 immunoprecipitates derived from WT or Fbw7−/− DLD1 cells. Anti-HA IgG was used as a negative control for the immunoprecipitation (IP) procedure.
Interestingly, depletion of cyclin E did not significantly change Rb phosphorylation or Cdt1 expression pattern (Figure 2B), indicating that the observed changes in the Cdh1 signaling pathway in cyclin E-depleted cells were not due to gross changes in the cell cycle or DNA replication process. Conversely, we found that overexpression of WT or a non-degradable form of cyclin E (T380A) in WT DLD1 cells could also lead to the aberrant APCCdh1 substrate expression similar to that observed in Fbw7−/− cells (Figure 2D and Supplementary information, Figure S2A). However, an elevation in Rb phosporylation, but not in the expression of Cdt1, was observed after cyclin E overexpression (Figure 2D). Nonetheless, as depletion of cyclin E reversed the aberrant APCCdh1 substrate expression in Fbw7−/− cells (Figure 2B), it suggests that cyclin E plays a central role in mediating the ability of Fbw7 to regulate APCCdh1 substrate abundance.
Consistently, both treatment with the Cdk2 inhibitor Roscovitine and depletion of Cdk2 via multiple independent shRNA vectors led to a sharp decrease in APCCdh1 substrates in Fbw7−/− cells (Figure 2E, 2F and Supplementary information, Figure S2B), suggesting that the kinase activity of Cdk2/cyclin E might be required for the inactivation of APCCdh1 in Fbw7−/− cells. It has been recently reported that Cdk2/cyclin E could phosphorylate Cdh1, leading to its dissociation from the APC core subunit25. Consistent with this report, we observed an obvious reduction in Cdh1 binding to the APC core subunit in Fbw7−/− cells (Figure 2G).
Fbw7 regulates the stability of various APCCdh1 substrates
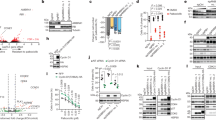
As Cdh1 is the activator of the APC/C E3 ligase, and the interaction between Cdh1 and the APC core subunit was reduced in Fbw7−/− cells (Figure 2G), we suggest that Fbw7 regulates Cdh1 and APCCdh1 substrates at a post-translational level. Consistently, we did not observe significant changes in the mRNA levels of Cdh1 (Figure 3A) or the APCCdh1 substrates Cdc20 (Figure 3B) and Skp2 (Figure 3C) in Fbw7−/− DLD1 cells compared with the WT DLD1 cells. On the other hand, using either Cycloheximide41,42 or Anisomycin43 to block protein synthesis as a means to measure protein stability, we observed an increase in the half-life of multiple APCCdh1 substrates including cyclin B, Plk1, cyclin A and Cdc20 (Figure 3D-3H, Supplementary information, Figure S3A, and S3D-S3H). These results indicate that increased protein stability, but not changes in mRNA levels, contributes to the elevated expression of various APCCdh1 substrates in Fbw7−/− cells.
Fbw7 negatively regulates the stability of various APCCdh1 substrates. (A-C) Real-time RT-PCR analysis to examine the relative Cdh1 (A), Cdc20 (B) and Skp2 (C) mRNA expression levels in WT vs Fbw7−/− DLD1 cells. Three independent sets of experiments were performed to generate the error bar. The error bars represent SD (D) WT or Fbw7−/− DLD1 cells were treated with 20 μg/ml cycloheximide (CHX). At the indicated time points, WCL were prepared and immunoblots were probed with the indicated antibodies. (E-H) Quantification of the band intensities in D. Cyclin B (E), Plk1 (F), cyclin A (G) and Cdc20 (H) band intensities were normalized to Tubulin, then normalized to the controls at 0 h. (I) Fbw7−/− DLD1 cells infected with the indicated shRNA lentiviral vectors followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells before being treated with 20 μg/ml CHX. At the indicated time points, WCL were prepared and immunoblots were probed with the indicated antibodies. (J-M) Quantification of the band intensities in I. Cyclin B (J), Plk1 (K), cyclin A (L) and Cdc20 (M) band intensities were normalized to Tubulin, then normalized to the controls at 0 h. (N) Immunoblot analysis of WCL and anti-HA immunoprecipitates derived from WT or Fbw7−/− HCT116 cells transfected with HA-Plk and Myc-ubiquitin. Forty hours post-transfection, 10 μM MG132 was added for 12 h before harvesting for IP assay. (O) Phosphorylation of Cdh1 by Cdk2/cyclin E inhibits the E3 ligase activity of APCCdh1 towards promoting cyclin B ubiquitination in vitro. Bacterially purified GST-NT-cyclin B proteins were incubated with the APC complex purified from G1-phase HeLa extracts together with purified E1, E2 and ubiquitin proteins at 30 °C for 60 min before being resolved by SDS-PAGE and probed with the anti-HA antibody. In vitro translated WT- or 4A-Cdh1 was added to activate the APC E3 ligase complex. Where indicated, WT- or 4A-Cdh1 was pre-incubated with the recombinant Cdk2/cyclin E prior to performing the in vitro ubiquitination assay.
Next, we found that depletion of cyclin E in Fbw7−/− cells led to a decrease in the half-life of APCCdh1 substrates including cyclin B, Plk1, cyclin A and Cdc20 (Figure 3I-3M, Supplementary information, Figure S3B and S3I-S3M), further supporting the critical role of cyclin E in mediating the ability of Fbw7 to regulate APCCdh1 substrate stability. Consistent with the role of Fbw7 in regulating APCCdh1 E3 ligase activity, we found that the ubiquitination of the APCCdh1 substrate Plk1 is significantly decreased in Fbw7−/− cells compared with that in WT cells (Figure 3N and Supplementary information, Figure S3C). More importantly, we found that pre-incubation with recombinant Cdk2 and cyclin E proteins dramatically decreased the ability of WT-Cdh1, but not that of the non-phosphorylatable 4A-Cdh1 mutant (Figure 4A and 4B), to activate the APC E3 ligase in the in vitro ubiquitination assays using cyclin B as the substrate (Figure 3O). This indicates that Cdk2/cyclin E might directly phosphorylate Cdh1, leading to its inactivation25 (Figure 6).
Cdk2/cyclin E phosphorylates Cdh1 at multiple sites, resulting in the inactivation of the APCCdh1 E3 ubiquitin ligase. (A) Schematic representation of the four major phosphorylation sites located in the N terminus of Cdh1 as well as in the GST-Cdh1 (N174) construct used in B. (B) Purified Cdk2/cyclin E kinase complex was incubated with 5 μg of indicated GST-Cdh1 in the presence of γ-32P-ATP. The kinase reaction products were resolved by SDS-PAGE and phosphorylation was detected by autoradiography. (C) Immunoblot analysis of WCL derived from HeLa cells transfected with Flag-Plk1 in the presence of WT- or 4A-Cdh1 together with the indicated WT- or T380A-cyclin E. (D) Immunoblot analysis of WCL derived from HeLa cells transfected with Flag-cyclin B in the presence of WT- or 4A-Cdh1 together with the indicated WT- or T380A-cyclin E. (E) Flag-Plk1 was cotransfected into HeLa cells with WT- or 4A-Cdh1 in the presence or absence of Cdk2/cyclin E. Forty hours post-transfection, the resulting cells were treated with 20 μg/ml CHX. At the indicated time points, WCL were prepared and immunoblots were probed with the indicated antibodies. (F) Fbw7−/− DLD1 cells were infected with a Tet-inducible lentiviral vector encoding Myc-WT-Cdh1 or Myc-4A-Cdh1, followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells before addition of doxycycline (Dox) for 16 h to induce the expression of Cdh1. Afterwards, the WCL were collected for immunoblot analysis with the indicated antibodies. (G) Immunoblot analysis of WCL and anti-Cdc27 immunoprecipitates derived from HeLa cells tranfected with the indicated Myc-Cdh1 expression constructs. (H) Immunoblot analysis of WCL and anti-Cdh1 immunoprecipitates derived from WT DLD1 colon cancer cells infected with a retrovirus vector encoding WT-cyclin E or T380A-cyclin E (with the pBabe-puro empty vector as a negative control). The cells were selected in 1 μg/ml puromycin for more than 72 h to eliminate the non-infected cells before the IP experiment. (I) Immunoblot analysis of WCL and anti-Cdh1 immunoprecipitates derived from Fbw7−/− DLD1 cells infected with the pLKO-based shcyclin E lentiviral vector (with shGFP as a negative control). The cells were selected in 1 μg/ml puromycin for more than 72 h to eliminate the non-infected cells before the IP experiment.
Cdk2/cyclin E directly phosphorylates Cdh1 at multiple sites, leading to the inactivation of the APCCdh1 E3 ubiquitin ligase
APCCdh1 is a master regulator of the G1/S cell cycle transition and thus its E3 ligase activity is tightly regulated. It was previously reported that phosphorylation of Cdh1 by Cdk2/cyclin A at multiple sites (S40, T121, S151 and S163) located at the N terminus of Cdh1 could lead to the dissociation of Cdh1 from the APC/C core subunit, thus terminating the E3 ligase activity of APCCdh1 before S phase entry44. Later work by Keck et al.25 expanded this finding by demonstrating that Cdk2/cyclin E could also phosphorylate Cdh1 to inactivate Cdh1. However, they did not completely specify the phosphorylation sites involved in the inactivation of Cdh1. To better understand the molecular mechanism by which cyclin E inactivates Cdh1, we performed in vitro kinase assays and defined that similar to Cdk2/cyclin A (Supplementary information, Figure S4A), Cdk2/cyclin E mainly phosphorylates Cdh1 at the four pinpointed sites (Figure 4A and 4B). Next, we performed in vivo degradation assays to explore how Cdk2/cyclin E-mediated phosphorylation of Cdh1 affects its ability to promote the destruction of the APCCdh1 substrates Plk1 and cyclin B. As illustrated in Figure 4C-4E, overexpression of WT-Cdh1 enhanced the destruction of both Plk1 and cyclin B, a process that can be inhibited by ectopic expression of Cdk2 and cyclin E. On the other hand, the ability of 4A-Cdh1 to enhance the destruction of either Plk1 or cyclin B was not affected by expression of either cyclin E or a non-degradable version of cyclin E (T380A) together with Cdk2. These results clearly demonstrated that elevated expression of cyclin E, which is observed in Fbw7-deficient cells, could lead to inactivation of the APCCdh1 E3 ligase and subsequent elevation of APCCdh1 substrates. As the mode of negative regulation of Cdh1 activity relies on Cdk2/cyclin E-dependent phosphorylation of Cdh1, the 4A-Cdh1 mutant that cannot be phosphorylated by Cdk2/cyclin E should maintain its ability to activate APC/C E3 ligase even in the Fbw7−/− genetic background. Consistent with this reasoning, we found that doxycycline-induced expression of 4A-Cdh1, but not WT-Cdh1, led to a decrease in the expression of various APCCdh1 substrates in Fbw7−/− DLD1 cells (Figure 4F, Supplementary information, Figure S4B and S4C).
Next, we further explored how Cdh1 phosphorylation caused by elevated Cdk2/cyclin E kinase activity in Fbw7−/− cells suppresses the E3 ligase activity of APCCdh1. As illustrated in Figure 4G, compared with WT or the 4A mutant form of Cdh1, the phospho-mimetic mutant form of Cdh1 (4D) displayed a significant reduction in association to the APC core subunit. More importantly, we further demonstrated that ectopic expression of cyclin E led to an increase in the phosphorylation of endogenous Cdh1 (Figure 4H), whereas depletion of cyclin E resulted in a sharp decrease in endogenous Cdh1 phosphorylation (Figure 4I). Therefore, consistent with previous reports25,44,45, these results support a model that the Fbw7/cyclin E signaling pathway potentially regulates the E3 ligase activity of APCCdh1 by affecting the interaction of Cdh1 with the APC core subunit primarily through regulating Cdh1 phosphorylation status (Figure 6).
Negative regulation of APCCdh1 E3 ligase activity by cyclin E contributes to the tumor suppressor function of Fbw7
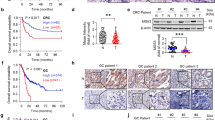
Next, we characterized the physiological significance of the signaling link between the Fbw7 and Cdh1 tumor suppressor pathways. Consistent with the tumor suppressor role of Fbw7, we found that loss of Fbw7 led to increased entry into the S phase, as illustrated by elevated BrdU labeling (Figure 5A and 5B). Furthermore, depletion of cyclin E (Figure 5A and 5B), or Cdk2 (Figure 5C and 5D) in Fbw7−/− cells resulted in a decreased BrdU labeling to levels comparable to those in WT cells. Thus, consistent with a previous report25, our work implicated Cdk2/cyclin E as a critical negative regulator of Cdh1 activity. We further defined that doxycycline-induced ectopic expression of 4A-Cdh1, but not WT-Cdh1, also led to a significant decrease in BrdU incorporation in Fbw7−/− cells (Figure 5E and 5F), suggesting that cyclin E functions in part through the Cdh1 signaling pathway to influence S phase entry. Additionally, we found that depletion of cyclin E in Fbw7−/− cells also led to a marked decrease in cell migration when compared with cells infected with shGFP as negative control (Supplementary information, Figure S5A). Notably, doxycycline-induced expression of 4A-Cdh1 is more efficient than WT-Cdh1 in suppressing anchorage-independent colony formation in Fbw7−/− cells (Figure 5G and 5H). However, this difference is not obvious in WT DLD1 colon cancer cells (Supplementary information, Figure S5B and S5C). These results indicate that elevated Cdk2/cyclin E-dependent phosphorylation of WT-Cdh1, but not 4A-Cdh1, in Fbw7−/− cells inactivates the tumor suppressor function of Cdh1. More importantly, using a xenograft mouse model, we showed that depletion of cyclin E in Fbw7−/− DLD1 cells significantly retarded their ability to form tumors in vivo (Figure 5I, 5J, Supplementary information, Figure S5D and S5E). Furthermore, consistent with the finding that depletion of cyclin E reduced Cdh1 phosphorylation (Figure 4I), we found that compared with tumors derived from mice injected with shGFP-treated cells, shcyclin E-treated Fbw7−/− DLD1 tumors exhibited a significant reduction in endogenous Cdh1 phosphorylation (Figure 5K). Together, these results suggest that Fbw7 regulates the Cdh1 tumor suppressor pathway through a cyclin E-dependent mechanism (Figure 6). Hence, loss of Fbw7 activity likely creates a scenario equal to loss of both the Fbw7 and Cdh1 tumor suppressor pathways, facilitating tumorigenesis.
Negative regulation of APCCdh1 E3 ligase activity by cyclin E contributes to the tumor suppressor function of Fbw7. (A) WT or the Fbw7−/− DLD1 cells were pulsed with BrdU for 30 min and then fixed for immunohistochemistry to analyze BrdU incorporation. Where indicated, the Fbw7−/− DLD1 cells were infected with an shcyclin E lentiviral vector (with an shGFP vector as a negative control) followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells before the BrdU labeling experiment. (B) Quantitation of the results obtained in A. (C) WT or the Fbw7−/− DLD1 cells were pulsed with BrdU for 30 min and then fixed for immunohistochemistry to analyze BrdU incorporation. Where indicated, the Fbw7−/− DLD1 cells were infected with multiple independent shCdk2 lentiviral vectors (with a shGFP vector as a negative control) followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells before the BrdU labeling experiment. (D) Quantitation of the results obtained in C. (E) WT or the Fbw7−/− DLD1 cells were pulsed with BrdU for 30 min and then fixed for immunohistochemistry to analyze the BrdU incorporation. Where indicated, Fbw7−/− DLD1 cells were infected with a Tet-inducible lentiviral vector encoding Myc-WT-Cdh1 or Myc-4A-Cdh1, followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells before addition of Dox for 16 h to induce the expression of Cdh1 before BrdU labeling. (F) Quantitation of the results obtained in E. (G) Fbw7−/− DLD1 cells were infected with a Tet-inducible lentiviral vector encoding Myc-WT-Cdh1 or Myc-4A-Cdh1 (or with empty vector as a negative control), followed by selection in 1 μg/ml puromycin for 72 h to eliminate the non-infected cells before addition of Dox for 16 h to induce the expression of Cdh1. Afterwards, the indicated cells were plated into soft agar-containing medium for anchorage-independent colony growth assays. Every 3-4 days, new fresh medium or Dox-containing medium was added as indicated. (H) Quantitation of the results obtained in G. In B, D, F and H, three independent sets of experiments were performed to generate the error bar. The error bars represent SD. (I) Growth curves for the xenograft experiments with the indicated Fbw7−/− DLD1 colon cancer cells that were inoculated subcutaneously. 2.5 × 106 cells were injected into the flank of 5 nude mice. The visible tumors were measured at the indicated days. Error bars represent SEM. P-value was calculated with the Student's t-test, *P < 0.05. (J) Mass of dissected tumors was measured and presented to illustrate that depletion of endogenous cyclin E retarded the in vivo tumorigenesis of Fbw7−/− DLD1 cells. (K) Immunoblot analysis of WCL and anti-Cdh1 immunoprecipitates derived from the tumors harvested from the xenograft assays performed in I and J.
Discussion
In this study, we present evidence for a possible model that loss of Fbw7 function promotes tumorigenesis by inactivating the APCCdh1 E3 ligase via cyclin E upregulation (Figure 6). In multiple colon cancer cell lines with a loss of the Fbw7 gene, elevated expression was observed not only for SCFFbw7 substrates, including cyclin E, but also for APCCdh1 substrates, including cyclin B, cyclin A, Plk1 and Skp2 (Figure 1B and Supplementary information, Figure S1B). Most importantly, this aberrant APCCdh1 substrate expression phenotype was rescued upon depletion of cyclin E in Fbw7−/− cells (Figure 2A and 2B), while overexpression of cyclin E in WT cells could faithfully phenocopy the aberrant APCCdh1 substrate expression found in Fbw7−/− cells (Figure 2D and Supplementary information, Figure S2A). These results suggest that cyclin E is a possible signaling link between these two tumor suppressor pathways that were previously thought to be mutually exclusive. Furthermore, these results also advocate for the usage of specific Cdk2/cyclin E inhibitors for treating Fbw7-deficient tumors. However, we also recognize that inactivation of Cdh1 by cyclin E might not be the only mechanism accounting for the tumor suppressor function of Fbw75,7, and more thorough studies are still required to fully elucidate other signaling pathways that may also play roles in mediating the tumor suppression function of Fbw7.
In addition to connecting the Fbw7 and Cdh1 tumor suppressor pathways, our work also further characterizes the molecular mechanism by which elevated levels of cyclin E can lead to APCCdh1 inactivation. While it was recently reported that phosphorylation of Cdh1 by Cdk2/cyclin E led to the dissociation of Cdh1 from the APC core subunit25, the exact phosphorylation sites involved in the regulation were not completely identified. Here we show that four sites present at the N terminus of Cdh1 (S40, T121, S151 and S163), which are the sites important for Cdk2/cyclin A-dependent regulation of APCCdh1 activity, are also critical for Cdk2/cyclin E-dependent negative regulation of Cdh1 (Figure 4B-4D). Furthermore, our findings extend the cyclin E-Cdh1 regulatory axis by including Fbw7 as an upstream component of this pathway (Figure 6). We also seek to demonstrate the physiological significance of the Fbw7-cyclin E-Cdh1 regulatory axis as frequent loss of Fbw7 is a major cause of cyclin E overexpression in human cancers. To this end, our results clearly show that depletion of cyclin E in Fbw7−/− cells retarded cell entry into S phase (Figure 5A and 5B). This is likely attributed to cyclin E-dependent inactivation of Cdh1 as depletion of cyclin E reversed the aberrant upregulation of APCCdh1 substrates (Figure 2B). Furthermore, doxycycline-induced expression of a non-phosphorylatable Cdh1 also resulted in delayed S phase entry (Figure 5E and 5F), mimicking the cyclin E-depletion phenotype. These findings therefore fill in the gap of knowledge regarding how Fbw7 governs the activity of Cdh1 to exert its anti-tumor functions.
With Cdh1 playing such a vital role in mediating the degradation of major cell cycle regulators, it is not surprising that Cdh1 was recently characterized to be a bona fide tumor suppressor26. Therefore, understanding how the tumor suppressor function of Cdh1 is regulated becomes increasingly more important. However, we recognize that as APCCdh1 has been reported to target numerous substrates, many of which are putative oncoproteins, for ubiquitination and degradation; further thorough studies are required to pinpoint the exact downstream target(s) of APCCdh1 that mediates its tumor suppressor function. Notably, in comparison to the frequent loss of Fbw7 in multiple types of human neoplasias, mutations in Cdh1 gene are not commonly observed46. Therefore, the signaling axis described in this study not only sheds lights on how Fbw7 functions as a tumor suppressor, but also on how Cdh1 is inactivated by means other than loss-of-function mutations or changes in Cdh1 protein abundance during cancer development. In this sense, loss of Fbw7 provides an umbrella effect that indirectly leads to the inactivation of APCCdh1. Thus our work provides a possible explanation for the lower frequency of Cdh1 mutations in human cancers. Similar overarching effects have also been observed for the p53/p21 and TSC2/mTOR signaling pathways47,48,49. Due to the high frequency of p53 and TSC2 mutations, p21 or mTOR mutations are much less frequent in human cancers. Furthermore, our findings also begin to highlight how Fbw7 can act as such a potent tumor suppressor, as loss of Fbw7 function would lead to the inhibition of two tumor suppressor pathways.
Materials and Methods
Expression constructs and shRNAs
HA-Fbw7 was obtained from Dr Keiichi I Nakayama (Kyushu University, Japan). HA-cyclinE-WT and HA-cyclin E-T380A were obtained from Dr Wade Harper (Harvard University). To construct pBabe-Puro-HA-cyclin E-WT and -T380A, human cyclin E-WT and -T380A cDNA was subcloned into the pBabe-Puro vector. Myc-Cdh1-WT and Myc-Cdh1-4A were obtained from Dr Jiri Lukas (Institute of Cancer Biology and Centre for Genotoxic Stress Research, Denmark). To generate the pGEX-Cdh1 truncation constructs (Cdh1-WT (N174) and Cdh1-4A (N174)) used in the in vitro kinase assays, truncated Cdh1 cDNA was amplified by PCR from Myc-Cdh1-WT or Myc-Cdh1-4A and the PCR product was subcloned into the pGEX-4T-1 vector. Flag-Plk1 and Flag-cyclin B were obtained from Dr Roya Khosravi-Far (Harvard University) and Dr Phil Hinds (Tufts University School of Medicine), respectively. HA-Cdk2 was obtained from Dr William Kaelin Jr (Dana-Farber Cancer Institute). The targeting sequences of pLKO lentiviral expression vectors to knock down human cyclin E and Cdk2 were obtained from The RNAi Consortium at the Broad Institute: shCycE_1 (TRCN0000045302), shCycE_2 (TRCN0000045298), shCdk2_1 (TRCN0000039958), shCdk2_2 (TRCN0000039959), and shCdk2_3 (TRCN0000039960).
Antibodies
α-Aurora A antibody (3092), α-pSer807pSer811-Rb antibody (9308), α-Cdt1 antibody (8064S), α-p27 antibody (3698P), α-p57 antibody (2557P) and α-pSP Cdk Substrate antibody (2324) were purchased from Cell Signaling. α-cyclin B1 antibody (GNS1), α-p130 antibody (C-20), α-p21 antibody (F-5), α-cyclin A antibody (H-432), α-Plk1 antibody (F-8), α-Cdc20 antibody (H-175), α-Cdc27 antibody (C-4), α-c-Myc antibody (9E10), polyclonal α-HA antibody (Y-11), α-cyclin E antibody (HE12), α-Cdc27 (C-4) agarose beads and α-p53 antibody (DO-1) were purchased from Santa Cruz Biotechnology. α-Tubulin antibody (T-5168), α-Vinculin antibody (V-4505), polyclonal α-Flag antibody (F-2425), monoclonal α-Flag antibody (F-3165), α-HA agarose beads (A-2095), peroxidase-conjugated α-mouse secondary antibody (A-4416) and peroxidase-conjugated α-rabbit secondary antibody (A-4914) were purchased from Sigma. Monoclonal α-HA antibody (MMS-101P) was purchased from Covance. α-GFP antibody (632380), monoclonal α-Skp2 antibody (32-3400), and polyclonal α-Cdh1 antibody (34-2000) were purchased from Invitrogen. Monoclonal α-Cdh1 (CC43) was purchased from Oncogene.
Cell culture and cell synchronization
Cell culture and cell synchronization experiments were performed as previously described41,50. Lentiviral shRNA packaging and infection were also performed as previously described51. The Cdk inhibitor roscovitine (Cell Signaling) was used as indicated. The T-ALL cell lines were generous gifts from Dr Ronald DePinho (MD Anderson Cancer Center) while the breast cancer cell lines were generous gifts from Dr Alex Toker (Harvard University). The WT-Fbw7 and Fbw7−/− colon cancer cell lines (DLD1 and HCT116) were kindly provided by Dr Bert Vogelstein (Johns Hopkins University).
Immunoblots and immunoprecipitation
Cells were lysed in EBC buffer (50mM Tris pH 7.5, 120mM NaCl, 0.5% NP-40) supplemented with protease inhibitors (Complete Mini, Roche) and phosphatase inhibitors (phosphatase inhibitor cocktail set I and II, Calbiochem). Protein concentrations were then measured using a Bio-Rad protein assay reagent on a Beckman Coulter DU-800 spectrophotometer. Finally, the lysates were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. For Cdc27 immunoprecipitation, 1 mg of lysate was incubated with anti-Cdc27 conjugated to agarose beads (Santa Cruz Biotechnology) overnight at 4 °C. Immunocomplexes were then washed 5 times with NETN buffer (20 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA and 0.5% NP-40) and resolved by SDS-PAGE and immunoblotted with the indicated antibodies.
In vitro kinase assay
For use in in vitro kinase assays, active Cdk2/cyclin E and Cdk2/cyclin A were purchased from Millipore and NEB, respectively. The protocol followed was previously described by Inuzuka et al.42. Briefly, 2.5 μg of the indicated GST fusion proteins were incubated with active Cdk2/cyclin E or Cdk2/cyclin A in the presence of 0.5 μCi [γ-32P] ATP and 200 μM cold ATP in Cdk2/cyclin kinase buffer. The reaction was allowed to proceed for 30 min before being stopped by the addition of SDS-containing loading buffer. The samples were then resolved by SDS-PAGE.
In vivo ubiquitination analysis
In vivo ubiquitination assays were performed as described previously42,52. Briefly, cells were transfected with plasmids encoding HA-Plk1 along with Myc-ubiquitin. Thirty-six hours after transfection, 10 μM MG132 was added to inhibit proteasomal degradation. Cells were then harvested in EBC buffer containing protease inhibitors and the collected whole-cell lysates (1 mg) were incubated with anti-HA beads (Sigma) or anti-Myc beads (Santa Cruz Biotechnology) for 4 h. After incubation, the precipitates were washed 6 times with NETN buffer, and the washed pellet was boiled in SDS-containing sample buffer and resolved by SDS-PAGE.
In vitro ubiquitination analysis
APCCdh1 in vitro ubiquitination assays were performed as described previously53. Briefly, 8 μg of anti-Cdc27 (AF3.1) antibodies coupled to 80 μl of protein A-agarose (Sigma) were incubated with 0.8 ml extracts of HeLa cells released 3 h from nocodazole arrest and mixed for 2 h at 4 °C. The beads were washed three times with 1 ml swelling buffer (SB, 25 mM HEPES, pH7.5, 1.5 mM MgCl2, 5 mM KCl) supplemented with 0.05% Tween 20 and twice with SB. Finally, the beads were resuspended in 40 μl of SB and aliquoted into 8 tubes (5 μl for each tube). Purified APC complexes were further activated by incubation with IVT-Cdh1 for 45 min as indicated. To generate the phosphorylated Cdh1, IVT-Cdh1 was treated with active cyclin E/Cdk2 and 100 μM ATP for 30 min at 30 °C prior to the incubation with APC complexes. 0.15 μM E1, 1 μM UBCH10, 1 mg/ml ubiquitin, Energy mix (7.5 mM creatine phosphate, 1 mM ATP, 1 mM MgCl2, 0.1 mM EGTA), and 1 U creatine phosphokinase were mixed in UBAB buffer (25 mM Tris/HCl, pH7.5, 50 mM NaCl, 10 mM MgCl2) in a final volume of 8 μl. Reactions were started by the addition of bacterially expressed and purified GST-cyclin B-NT (1-119) as substrate, incubated for 60 min at 30 °C, resolved by SDS-PAGE and immunoblotted with the GST antibody.
Cell migration assay
Cell migration assays were performed as previously described54. Briefly, 1 × 105 cells in serum-free media containing 0.1% BSA were added to the upper chamber of a Transwell Filter (8 μm pore size; Corning) in triplicates. NIH 3T3-cell-conditioned media was added to the lower chamber. After a 16-h incubation at 37 °C, non-migrated cells at the top of the filter were removed using a cotton swab. Cells that had migrated to the bottom of the filter were fixed and stained using the Hema-3 stain set (purchased from Protocol). Cells were then counted using a 20× objective, and four fields were chosen per well with three wells per each condition.
Bromodeoxyuridine (BrdU) labeling
BrdU labeling was performed as described previously55. The experiments were performed 3 times to generate error bars.
Soft agar colony formation assay
Soft agar growth assays were performed as described previously56. Briefly, 24 h before plating, cells were treated with 100 ng/ml of doxycycline to induce expression of the various Cdh1 constructs. At the time of plating in soft agar, cultures were trypsinized and counted, and 1 × 104 total cells were mixed with 1.5 ml of 0.4% Noble agar-DMEM (top layer) and then poured on top of 5 ml of solidified 0.8% Noble agar-DMEM (bottom layer) in 6-cm diameter dishes. Cells were fed weekly by overlaying 1 ml of growth media supplemented with doxycycline as indicated. After 3 weeks, colonies were stained with 1 mg/ml iodomi tetrazolium chloride, counted and pictures were taken. The experiment was performed three times to generate error bars.
Real-time RT-PCR analysis
RNA was extracted with a Qiagen RNeasy mini kit, and the reverse transcription reaction was performed with ABI Taqman Reverse Transcriptional Reagents (N808-0234). After mixing the resulting template with ABI Taqman Fast Universal PCR Master Mix (4352042) and Cdh1 (FZR1) (Hs00393592_m1), Cdc20 (Hs00415851_g1), or Skp2 (Hs00180634_m1) primers, the real-time RT-PCR reaction was performed on an ABI-7500 Fast Real-time PCR system.
FACS analysis
FACS analysis was performed as previously described50.
Xenograft mouse model
In vivo tumorigenesis assays were performed as previously described42. Briefly, 2.5 × 106 cells were injected into the hind flank of male nude mice. Tumor size was measured every 3-4 days after initial tumor growth using a caliper. The tumor volume was then determined using the formula: L × W2 × 0.52, where L is the longest diameter and W is the shortest diameter of the measured growth.
References
Bakhoum SF, Compton DA . Cancer: CINful centrosomes. Curr Biol 2009; 19:R642–R645.
Bannon JH, Mc Gee MM . Understanding the role of aneuploidy in tumorigenesis. Biochem Soc Trans 2009; 37:910–913.
Schvartzman JM, Sotillo R, Benezra R . Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat Rev Cancer 2010; 10:102–115.
Nakayama KI, Nakayama K . Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer 2006; 6:369–381.
Cardozo T, Pagano M . The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol 2004; 5:739–751.
Nakayama KI, Nakayama K . Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin Cell Dev Biol 2005; 16:323–333.
Welcker M, Clurman BE . FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 2008; 8:83–93.
Spruck CH, Strohmaier H, Sangfelt O, et al. hCDC4 gene mutations in endometrial cancer. Cancer Res 2002; 62:4535–4539.
Bai C, Sen P, Hofmann K, et al. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 1996; 86:263–274.
Petroski MD, Deshaies RJ . Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 2005; 6:9–20.
Hao B, Oehlmann S, Sowa ME, Harper JW, Pavletich NP . Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol Cell 2007; 26:131–143.
Orlicky S, Tang X, Willems A, Tyers M, Sicheri F . Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell 2003; 112:243–256.
Minella AC, Clurman BE . Mechanisms of tumor suppression by the SCF(Fbw7). Cell Cycle 2005; 4:1356–1359.
Akhoondi S, Sun D, von der Lehr N, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res 2007; 67:9006–9012.
Enders GH . Cyclins in breast cancer: too much of a good thing. Breast Cancer Res 2002; 4:145–147.
Malyukova A, Dohda T, von der Lehr N, et al. The tumor suppressor gene hCDC4 is frequently mutated in human T-cell acute lymphoblastic leukemia with functional consequences for Notch signaling. Cancer Res 2007; 67:5611–5616.
Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI . Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 2001; 413:316–322.
Thompson BJ, Buonamici S, Sulis ML, et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J Exp Med 2007; 204:1825–1835.
O'Neil J, Grim J, Strack P, et al. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J Exp Med 2007; 204:1813–1824.
Onoyama I, Tsunematsu R, Matsumoto A, et al. Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis. J Exp Med 2007; 204:2875–2888.
Rajagopalan H, Jallepalli PV, Rago C, et al. Inactivation of hCDC4 can cause chromosomal instability. Nature 2004; 428:77–81.
Minella AC, Swanger J, Bryant E, Welcker M, Hwang H, Clurman BE . p53 and p21 form an inducible barrier that protects cells against cyclin E-cdk2 deregulation. Curr Biol 2002; 12:1817–1827.
Loeb KR, Kostner H, Firpo E, et al. A mouse model for cyclin E-dependent genetic instability and tumorigenesis. Cancer Cell 2005; 8:35–47.
Zhang H . Life without kinase: cyclin E promotes DNA replication licensing and beyond. Mol Cell 2007; 25:175–176.
Keck JM, Summers MK, Tedesco D, et al. Cyclin E overexpression impairs progression through mitosis by inhibiting APC(Cdh1). J Cell Biol 2007; 178:371–385.
Garcia-Higuera I, Manchado E, Dubus P, et al. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol 2008; 10:802–811.
Burton JL, Solomon MJ . D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev 2001; 15:2381–2395.
Pfleger CM, Kirschner MW . The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev 2000; 14:655–665.
Li M, Zhang P . The function of APC/CCdh1 in cell cycle and beyond. Cell Div 2009; 4:2.
Skaar JR, Pagano M . Cdh1: a master G0/G1 regulator. Nat Cell Biol 2008; 10:755–757.
Wasch R, Engelbert D . Anaphase-promoting complex-dependent proteolysis of cell cycle regulators and genomic instability of cancer cells. Oncogene 2005; 24:1–10.
Wasch R, Robbins JA, Cross FR . The emerging role of APC/CCdh1 in controlling differentiation, genomic stability and tumor suppression. Oncogene 2010; 29:1–10.
Engelbert D, Schnerch D, Baumgarten A, Wasch R . The ubiquitin ligase APC(Cdh1) is required to maintain genome integrity in primary human cells. Oncogene 2008; 27:907–917.
Ross KE, Cohen-Fix O . The role of Cdh1p in maintaining genomic stability in budding yeast. Genetics 2003; 165:489–503.
Wang X, Di K, Zhang X, et al. Id-1 promotes chromosomal instability through modification of APC/C activity during mitosis in response to microtubule disruption. Oncogene 2008; 27:4456–4466.
Shim EH, Johnson L, Noh HL, et al. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res 2003; 63:1583–1588.
Gstaiger M, Jordan R, Lim M, et al. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA 2001; 98:5043–5048.
Bischoff JR, Anderson L, Zhu Y, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J 1998; 17:3052–3065.
Eckerdt F, Yuan J, Strebhardt K . Polo-like kinases and oncogenesis. Oncogene 2005; 24:267–276.
Liao C, Wang XY, Wei HQ, et al. Altered myelopoiesis and the development of acute myeloid leukemia in transgenic mice overexpressing cyclin A1. Proc Natl Acad Sci USA 2001; 98:6853–6858.
Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W . Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol 2009; 11:397–408.
Inuzuka H, Tseng A, Gao D, et al. Phosphorylation by casein kinase I promotes the turnover of the Mdm2 oncoprotein via the SCF(beta-TRCP) ubiquitin ligase. Cancer Cell 2010; 18:147–159.
Gao C, Guo H, Downey L, Marroquin C, Wei J, Kuo PC . Osteopontin-dependent CD44v6 expression and cell adhesion in HepG2 cells. Carcinogenesis 2003; 24:1871–1878.
Lukas C, Sorensen CS, Kramer E, et al. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 1999; 401:815–818.
Hsu JY, Reimann JD, Sorensen CS, Lukas J, Jackson PK . E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat Cell Biol 2002; 4:358–366.
Qiao X, Zhang L, Gamper AM, Fujita T, Wan Y . APC/C-Cdh1: from cell cycle to cellular differentiation and genomic integrity. Cell Cycle 2010; 9:3904–3912.
Levine AJ . p53, the cellular gatekeeper for growth and division. Cell 1997; 88:323–331.
Riley T, Sontag E, Chen P, Levine A . Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 2008; 9:402–412.
Sabatini DM . mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 2006; 6:729–734.
Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG, Jr . Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 2004; 428:194–198.
Boehm JS, Hession MT, Bulmer SE, Hahn WC . Transformation of human and murine fibroblasts without viral oncoproteins. Mol Cell Biol 2005; 25:6464–6474.
Lu K, Yin X, Weng T, et al. Targeting WW domains linker of HECT-type ubiquitin ligase Smurf1 for activation by CKIP-1. Nat Cell Biol 2008; 10:994–1002.
Wu T, Merbl Y, Huo Y, Gallop JL, Tzur A, Kirschner MW . UBE2S drives elongation of K11-linked ubiquitin chains by the anaphase-promoting complex. Proc Natl Acad Sci USA 2010; 107:1355–1360.
Chin YR, Toker A . The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell 2010; 38:333–344.
Gao D, Inuzuka H, Korenjak M, et al. Cdh1 regulates cell cycle through modulating the claspin/Chk1 and the Rb/E2F1 pathways. Mol Biol Cell 2009; 20:3305–3316.
Bazarov AV, Adachi S, Li SF, Mateyak MK, Wei S, Sedivy JM . A modest reduction in c-myc expression has minimal effects on cell growth and apoptosis but dramatically reduces susceptibility to Ras and Raf transformation. Cancer Res 2001; 61:1178–1186.
Acknowledgements
We would like to thank Shavali Shaik and Zhiwei Wang for critical reading of the manuscript, and William Hahn for providing reagents. WW is an MLSC New Investigator, ACS Research Scholar, LLS Research Scholar and DOD Prostate Cancer Program New Investigator, and AWL is an NRSA T32 postdoctoral fellow. This work was supported in part by the NIH grants (WW, GM089763 and GM094777; YS, CA118762 and CA156744).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Depletion of Fbw7 leads to aberrant elevation of various Cdh1 downstream substrates. (PDF 180 kb)
Supplementary information, Figure S2
Cyclin E plays an important role in mediating the ability of Fbw7 to regulate Cdh1 substrate abundance. (PDF 111 kb)
Supplementary information, Figure S3
The Fbw7/Cyclin E signaling axis negatively regulates the stability of various Cdh1 substrates. (PDF 215 kb)
Supplementary information, Figure S4
Phosphorylation of Cdh1 by Cdk2/Cyclin E at multiple sites results in the inactivation of the APC/Cdh1 E3 ubiquitin ligase. (PDF 128 kb)
Supplementary information, Figure S5
Cyclin E-dependent suppression of Cdh1 E3 ligase activity contributes to the tumor suppressor function of Fbw7. (PDF 312 kb)
Rights and permissions
About this article
Cite this article
Lau, A., Inuzuka, H., Fukushima, H. et al. Regulation of APCCdh1 E3 ligase activity by the Fbw7/cyclin E signaling axis contributes to the tumor suppressor function of Fbw7. Cell Res 23, 947–961 (2013). https://doi.org/10.1038/cr.2013.67
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2013.67