Abstract
Monocytes are mobilized to sites of infection via interaction between the chemokine MCP-1 and its receptor, CCR2, at which point they differentiate into macrophages that mediate potent antimicrobial effects. In this study, we investigated the mechanisms by which monocytes are mobilized in response to systemic challenge with the intracellular bacterium Francisella tularensis. We found that mice deficient in MyD88, interferon-γ (IFNγ)R or CCR2 all had defects in the expansion of splenic monocyte populations upon F. tularensis challenge, and in control of F. tularensis infection. Interestingly, MyD88-deficient mice were defective in production of IFNγ, and IFNγR-deficient mice exhibited defective production of MCP-1, the ligand for CCR2. Transplantation of IFNγR-deficient bone marrow (BM) into wild-type mice further suggested that mobilization of monocytes in response to F. tularensis challenge required IFNγR expression on BM-derived cells. These studies define a critical host defense circuit wherein MyD88-dependent IFNγ production signals via IFNγR expressed on BM-derived cells, resulting in MCP-1 production and activation of CCR2-dependent mobilization of monocytes in the innate immune response to systemic F. tularensis challenge.
Similar content being viewed by others
Introduction
The mammalian organism is confronted at all times with a vast microbial menagerie consisting of a range of bacterial species, some of which are pathogenic and must be eliminated from the host. The mammalian immune system has developed a specialized set of immune mechanisms to detect and combat these pathogenic microorganisms 1. The initial immune response is mediated by innate immunity, which is characterized by the rapid induction of an inflammatory response designed to control infection and activate the subsequent adaptive response. The innate immune response is induced via the recognition of pathogen-associated molecular patterns by an array of pattern recognition receptors such as the Toll-like receptors (TLRs) and NOD-like receptors (NLRs) 2, 3. These in turn lead to the activation of an array of cytokines, chemokines, adhesion molecules and antimicrobial peptides that result in proinflammatory immune responses that promote elimination of the pathogen 2, 4.
Upon pathogen recognition, a defining feature of the innate immune response is the mobilization and trafficking of myeloid cells, including neutrophils and monocytes, to infected tissues, where they phagocytose and kill pathogenic microbes 5. In particular, monocytes have been shown to play a critical role in the control and elimination of intracellular bacteria such as Listeria monocytogenes, which has been extensively used as a model pathogen for studying the innate immune response to intracellular bacterial infection 6. Mice deficient in CCR2, the receptor for the monocyte chemokines MCP-1 and MCP-3, fail to mobilize monocyte populations in response to infection by intracellular bacteria and have impaired bacterial clearance 7, 8, 9. In response to L. monocytogenes infection, Ly6C+ monocytes accumulate in the spleen, where they mature into TNF-α- and iNOS-producing inflammatory monocytes 10. These cells in turn act as antimicrobial effector cells and can promote the generation of adaptive T-cell responses 10.
Activation of effective monocyte responses to intracellular bacteria such as F. tularensis is enhanced via production of interferon-γ (IFNγ) by activated NK and NKT cells early on, and subsequently by antigen-specific activation of CD4+ Th1 cell subsets 11, 12, 13. IFNγ has been shown to induce antimicrobial genes such as iNOS and LRG47, as well as to promote nutrient sequestration and the expression of antigen presentation molecules in multiple cell types 14, 15. Although the role of IFNγ in the activation of antimicrobial genes in monocytes is well characterized, a potential role of IFNγ in regulating other innate immune system functions, particularly the mobilization of myeloid cells, is not well defined.
In the present study, the mechanism by which monocytes are mobilized to sites of infection, in particular the spleen, after systemic Francisella tularensis challenge was investigated. F. tularensis is a gram-negative, intracellular bacterium that is the causative agent of tularemia, a potentially fatal zoonosis in humans if inoculation occurs via the pulmonary route 16. Recognition of F. tularensis by the innate immune system is largely mediated by TLR2 and MyD88 16, 17 and requires myeloid cells, including monocytes, as depletion of myeloid cells in mice using an anti-Gr-1 antibody (which recognizes the monocyte surface marker Ly6C as well as the granulocyte marker Ly6G) results in uncontrolled infection 18. Further, resolution of F. tularensis infection requires IFNγ signaling and the activation of antimicrobial genes including TNF-α and iNOS 19, 20, 21, 22.
Although prior work has suggested that IFNγ production plays a role in the formation of hepatic granulomas in mice upon F. tularensis challenge, the mechanism by which IFNγ mediates myeloid cell trafficking, and the identity and the functional importance of the mobilized cells have not been established 23. In the present study, we demonstrate that IFNγR signaling in bone marrow (BM)-derived cells is required for the production of the monocyte chemokine MCP-1 and mobilization of monocyte populations in the spleen following bacterial challenge. Furthermore, we show the production of IFNγ and resultant MCP-1 in response to systemic F. tularensis challenge occurs via an MyD88-dependent mechanism. Lastly, effective control of F. tularensis infection requires TNF-α and NO production by the IFNγR/CCR2-mobilized monocytes. Taken together, this research identifies a new MyD88-dependent signaling mechanism proceeding via IFNγR/CCR2 that governs the mobilization and activation of monocytes in response to challenge with a systemic intracellular bacterium.
Results
IFNγR expression is required for MCP-1 production and expansion of monocyte populations in the spleen after systemic F. tularensis challenge
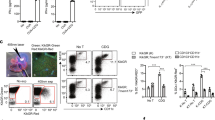
IFNγR signaling is a crucial mediator of immune responses to intracellular bacterial pathogens, including F. tularensis 19, 24. Thus, we asked whether IFNγR signaling participates not only in the activation of antimicrobial mechanisms but also in the mobilization of monocytes during intracellular bacterial challenge using an established systemic F. tularensis infection model in mice 19, 25. Wild-type (WT) and IFNγR-deficient mice were inoculated intravenously with 2 × 104 colony-forming units (CFUs) of F. tularensis, and, consistent with prior published results 19, 25, IFNγR-deficient mice but not WT mice were highly susceptible to this F. tularensis challenge (Supplementary information, Figure S1). These data indicate that IFNγR signaling is critical for host defense against F. tularensis. To assess how IFNγR signaling affects monocyte populations in the spleen after infection, we harvested spleens from infected WT and IFNγR-deficient mice and from uninfected control mice 24 h after F. tularensis challenge (Figure 1A-1C). We observed a significant expansion of CD11b+ Ly6Chi monocytes in the spleens of WT mice 24 h after infection. In contrast, there was no increase in splenic monocytes in IFNγR-deficient mice relative to uninfected controls. On the other hand, IFNαβR-deficient mice did not exhibit susceptibility to infection or defective expansion of monocyte populations after inoculation with F. tularensis, indicating that type I interferons are not required for host defense against F. tularensis (Supplementary information, Figure S1 and data not shown). These findings suggest that IFNγR signaling is crucial for the expansion of splenic monocyte populations after systemic F. tularensis challenge.
IFNγR expression is required for MCP-1 production and expansion of monocyte populations in the spleen after systemic F. tularensis challenge. WT and IFNγR−/− mice (n = 3/grp) were inoculated intravenously with 2 × 104 CFU F. tularensis. After 24 h, infected mice were euthanized along with uninfected (0 h) controls (n = 3/grp). (A) Representative analysis dot plot of RBC-depleted total splenocytes showing monocytes (CD11b+, Ly6Chi population) in control and F. tularensis-infected WT and IFNγR−/− mice. Monocyte populations are shown as (B) mean proportion of total splenocytes ± SD obtained from dot plots described in (A). (C) Mean absolute number of splenic monocytes ± SD. (D) Mean mRNA levels ± SD of MCP-1 from control and infected WT and IFNγR−/− mice. (E) Mean protein levels ± SD of MCP-1 from spleen homogenates and sera of WT and IFNγR-deficient mice. *p < 0.05 WT versus IFNγR−/− mice. Data are representative of at least five repeated experiments.
Previous work has described a critical role for the monocyte chemokine MCP-1 in mobilizing monocytes to the periphery in response to systemic bacterial challenge. As there was a defective expansion of monocyte populations in the spleens of IFNγR-deficient mice in response to systemic F. tularensis challenge (Figure 1A-1C), we hypothesized that this may be related to defective MCP-1 production in these mice. We therefore determined the induction of MCP-1 mRNA in the spleens of WT mice, IFNγR-deficient mice, and uninfected control mice by Q-PCR 24 h after F. tularensis inoculation. There was a profound impairment in MCP-1 mRNA induction in the spleens of IFNγR-deficient mice compared with WT mice after F. tularensis infection (Figure 1D). Similarly, MCP-1 protein production as measured by ELISA was also severely impaired in the spleens and sera of IFNγR-deficient mice compared with WT control mice after F. tularensis infection (Figure 1E). Collectively, these data reveal a crucial role for IFNγR signaling in the activation of MCP-1 production and the subsequent expansion of splenic monocyte populations.
IFNγR expression in the BM compartment is necessary for MCP-1 production and expansion of monocyte populations in the spleen after systemic F. tularensis challenge
IFNγR is expressed in a wide range of tissues, including BM-derived and non-BM-derived cell types. Therefore, we wanted to determine which compartment utilized IFNγR signaling to regulate expansion of splenic monocyte populations in response to systemic F. tularensis infection. WT recipient mice were lethally irradiated and reconstituted with either WT or IFNγR-deficient BM, generating WT BM→WT and IFNγR-deficient BM→WT chimeric mice. Eight weeks after reconstitution, these mice were inoculated intravenously with F. tularensis. WT BM→WT mice had increased proportions of monocytes in their spleens 24 h after infection; in contrast, IFNγR-deficient BM→WT mice had virtually no expansion of splenic monocytes (Figure 2A).
IFNγR expression in the bone marrow compartment is necessary for MCP-1 production and expansion of monocyte populations in the spleen after systemic F. tularensis challenge. WT BM→WT and IFNγR−/− BM→WT mice (n = 3/grp) were inoculated intravenously with 2 × 104 CFU F. tularensis. After 24 h, infected mice were euthanized along with uninfected controls (n = 3/grp). (A) Mean proportion of splenic monocytes (CD11b+, Ly6Chi population) ± SD was determined as in Figure 1A in control and F. tularensis-infected WT BM→WT and IFNγR−/− BM→WT mice. (B) Mean protein levels ± SD of MCP-1 from spleen homogenates and sera of WT and IFNγR-deficient mice. (C) F. tularensis CFU recovered from spleens of WT BM→WT and IFNγR−/− BM→WT mice (n = 3/grp) three days after inoculation with 2 × 104 CFU F. tularensis. *p < 0.05 WT BM→WT versus IFNγR−/− BM→WT mice, ‡p < 0.01 WT BM→WT versus IFNγR−/− BM→WT mice. Data are representative of at least three repeated experiments.
Further, a similar defect in splenic MCP-1 levels in F. tularensis-infected IFNγR-deficient BM→WT chimeric mice was observed compared with WT BM→WT control mice (Figure 2B). These data suggest that IFNγR expression in BM-derived cells is required for MCP-1 production and the mobilization of monocyte populations in response to F. tularensis challenge. Similar to IFNγR-deficient mice, IFNγR-deficient BM→WT chimeric mice had a substantially increased bacterial burden in the spleen, suggesting that BM expression of IFNγR is also required for host defense against F. tularensis (Figure 2C).
IFNγR signaling induces MCP-1 production in BM-derived cells and acts synergistically with innate sensing mechanisms to induce MCP-1 in vitro
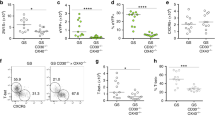
Our data suggest that BM expression of IFNγR is necessary for the production of MCP-1 in response to systemic F. tularensis challenge. MCP-1 can be produced by monocytes/macrophages, which arise from the BM 26, 27, 28. Thus, we evaluated the ability of IFNγ to induce MCP-1 production, using primary BM-derived macrophages (BMMs) as a model. Treatment of BMMs with IFNγ resulted in significantly increased MCP-1 mRNA expression as determined by QPCR (Figure 3A). Furthermore, the effect of IFNγ on MCP-1 protein production was dose-dependent, as determined by ELISA 24 h after stimulation (Figure 3B).
IFNγR signaling induces MCP-1 production in bone marrow-derived cells and acts synergistically with innate sensing mechanisms to induce increased levels of MCP-1 in vitro. ((A) Mean MCP-1 mRNA levels ± SD from WT control BMMs and WT BMMs stimulated with 0.1 ng/ml IFNγ for 6 h prior to harvest. (B) Mean MCP-1 protein levels ± SD from supernatants of WT control BMMs and WT BMMs stimulated with the indicated concentrations of IFNγ for 24 h prior to harvest. (C) Mean MCP-1 mRNA levels from control BMMs and BMMs stimulated with 1 ng/ml IFNγ, 0.01 ng/ml Pam3CysK, or both for 6 h prior to harvest. (D) Mean MCP-1 protein levels from supernatants of control BMMs and BMMs stimulated with 1 ng/ml IFNγ, MOI 1 F. tularensis, or both for 24 h prior to harvest. *p < 0.05 versus media control, **p < 0.05 versus media control and other data points. Data are representative of at least three repeated experiments.
During systemic infection, monocytes and macrophages in the spleen likely encounter both F. tularensis and IFNγ simultaneously. As pattern recognition receptors such as the TLRs also activate MCP-1 expression, we assessed whether IFNγR and TLRs interact in the induction of MCP-1 expression. As F. tularensis can be recognized via TLR2 16, BMMs were stimulated with the TLR2 ligand Pam3CysK in the presence or absence of IFNγ. Interestingly, a synergistic induction of MCP-1 mRNA was observed in BMMs treated with both Pam3CysK and IFNγ (Figure 3C). Furthermore, to more closely simulate our in vivo bacterial challenge model, BMMs were cultured with F. tularensis in the presence or absence of IFNγ and culture supernatants were harvested at 24 h for analysis of MCP-1 levels by ELISA. F. tularensis infection alone induced a modest amount of MCP-1 protein. However, addition of IFNγ to the F. tularensis-stimulated BMM cultures induced a synergistic increase in MCP-1 production, which was similar to our results with the TLR2 ligand (Figure 3D). Taken together, these data suggest that IFNγ is sufficient to activate MCP-1 production in BMMs. Furthermore, the IFNγ-dependent activation of MCP-1 can be amplified by TLR2 or F. tularensis stimulation.
MyD88-dependent signaling is required for IFNγ-driven MCP-1 production and expansion of splenic monocyte populations in response to systemic F. tularensis challenge
Previously published work has demonstrated that the adaptor protein MyD88, which is utilized by IL-1R, IL-18R, and most TLRs to initiate signaling, is a critical mediator of host defense against F. tularensis 29, 30. Consistent with these findings, we observed that MyD88-deficient mice are highly susceptible to systemic F. tularensis challenge and cannot effectively clear bacteria (Supplementary information, Figure S2). However, we specifically wanted to ascertain whether MyD88 was required for the IFNγR-dependent MCP-1 production and expansion of splenic monocytes. After systemic F. tularensis challenge, MyD88-deficient mice had the same defect in expansion of splenic monocyte populations (Figure 4A) as we previously observed in IFNγR-deficient mice (Figure 1A and 1C). Furthermore, we found that MCP-1 protein levels in the spleens of infected MyD88-deficient mice were substantially decreased compared with WT mice (Figure 4B). Given these findings, we hypothesized that MyD88 may either be required for production of IFNγ or that it may be necessary for production of MCP-1 via a different mechanism that does not impact IFNγ production. Therefore, IFNγ levels in the sera of WT and MyD88-deficient mice were measured after systemic challenge with F. tularensis. Consistent with prior published results 31, there was no detectable IFNγ in the sera of infected MyD88-deficient mice compared to robust IFNγ production in WT mice (Figure 4C). These findings suggest that the production of IFNγ is dependent upon MyD88-mediated signals.
IFNγ-driven MCP-1 expression and expansion of splenic monocyte populations in response to systemic F. tularensis challenge occurs via MyD88-dependent signaling. WT and MyD88−/− mice (n = 3/grp) were inoculated intravenously with 2 × 104 CFU F. tularensis. After 24 h, infected mice were euthanized along with uninfected (0 h) controls (n = 3/grp). (A) Mean proportion of splenic monocytes (CD11b+, Ly6Chi population) ± SD was determined as in Figure 1A in control and F. tularensis-infected WT and MyD88−/− mice. (C) Mean mRNA levels ± SD of MCP-1 from control and infected WT and MyD88−/− mice. (D) Mean protein levels ± SD of IFNγ in sera from control and infected WT and MyD88−/− mice. ND, not detected. *p < 0.05 WT versus MyD88−/− mice (Student's t-test). Data are representative of at least three repeated experiments.
CCR2-deficient mice exhibit defective expansion of splenic monocyte populations and host defense in response to F. tularensis challenge
To directly test whether IFNγR-dependent MCP-1 production plays an important role in the mobilization of monocytes to the spleen in the context of systemic F. tularensis challenge in vivo, we examined the expansion of CD11b+ Ly6Chi monocyte populations in the spleens of CCR2-deficient mice. These mice lack the receptor for MCP-1 and are thus unable to mobilize monocytes to the periphery upon bacterial challenge 7, 31, 32. Similar to IFNγR-deficient mice, we observed defective expansion of splenic monocytes in CCR2-deficient mice after F. tularensis challenge (Figure 5A). These findings directly link MCP-1 to the IFNγ-dependent splenic monocyte expansion observed in vivo. We then hypothesized that if the monocyte populations mobilized to the spleen are critical to host defense against F. tularensis, then infected CCR2-deficient mice should have increased bacterial CFU in their spleens. We therefore challenged WT, IFNγR-deficient, and CCR2-deficient mice with F. tularensis, and subsequently assessed bacterial CFU in spleens from these mice. Notably, there were approximately tenfold more F. tularensis CFU in the spleens of CCR2-deficient mice compared with WT control mice three days after inoculation, indicating that CCR2-driven mobilization of monocytes to the spleen is required for control of bacterial numbers (Figure 5B). Interestingly, we observed tenfold more bacterial CFU in the spleens of IFNγR-deficient mice than in CCR2-deficient mice, suggesting that IFNγR is required for additional host defense functions aside from driving the CCR2-dependent expansion of splenic monocytes (Figure 5B). Taken together, these observations define a host defense circuit in which IFNγR signaling in BM-derived cells activates production of MCP-1, which is in turn required for the mobilization of monocytes to the spleen and effective control of F. tularensis numbers there.
CCR2-deficient mice exhibit defective expansion of splenic monocyte populations and host defense in response to systemic F. tularensis challenge. WT mice, IFNγR−/− mice, and CCR2−/− mice (n = 3/grp) were inoculated intravenously with 2 × 104 CFU F. tularensis. After 24 h, infected mice were euthanized along with uninfected controls (n = 3/grp). (A) Mean proportion of splenic monocytes (CD11b+, Ly6Chi population) ± SD was determined as in Figure 1A in control and F. tularensis-infected WT mice, IFNγR−/− mice, and CCR2−/− mice. (B) F. tularensis CFU recovered from the spleens of WT, IFNγR−/− mice, and CCR2−/− mice three days after intravenous inoculation with 2 × 104 CFU F. tularensis. *p < 0.05 WT versus IFNγR−/− or CCR2−/− mice. Data are representative of at least three repeated experiments.
IFNγR-deficient and CCR2-deficient mice have severe defects in bacterial clearance and in production of TNF-α and nitric oxide following systemic F. tularensis challenge
Prior work has suggested that upon reaching areas of infection, monocytes produce TNF-α and NO, critical effector mechanisms for clearing intracellular bacterial pathogens such as F. tularensis 10. Thus, we examined whether CCR2-dependent mobilization of monocytes to the spleen was required for TNF-α and NO production in response to F. tularensis. Thus, we harvested spleens from WT, IFNγR-deficient and CCR2-deficient mice 24 h after F. tularensis challenge to assess production of TNF-α and NO. We found that intracellular TNF-α production in CD11b+ splenocytes harvested from F. tularensis-infected IFNγR-deficient and CCR2-deficient mice was severely impaired compared with the TNF-α production in WT control mice (Figure 6A). Furthermore, to determine whether these splenocytes were capable of producing NO, cells from F. tularensis-challenged WT, IFNγR-deficient, and CCR2-deficient mice were cultured ex vivo with heat-killed F. tularensis (HKFT), and culture supernatants were evaluated for production of nitrite, a NO breakdown product. Similar to TNF-α levels, there were significantly lower levels of nitrite in the supernatants of HKFT-stimulated splenocytes from IFNγR-deficient and CCR2-deficient mice relative to WT mice (Figure 6B). Taken together, these data suggest that IFNγR-dependent CCR2 signaling is required for the mobilization of monocytes to the spleen, where they act as critical producers of TNF-α and NO and mediate clearance of F. tularensis infection.
Mice deficient in CCR2 have similar defects as IFNγR-deficient mice in production of TNF-α and nitric oxide by inflammatory monocytes following systemic F. tularensis challenge. WT, IFNγR−/− mice, and CCR2−/− mice (n = 3/grp) were inoculated intravenously with 2 × 104 CFU F. tularensis. (A) Mean proportion ± SD of CD11b+ splenocytes producing TNF-α by intracellular staining from WT mice, IFNγR−/− mice, and CCR2−/− mice. (B) Mean nitrite production by splenocytes harvested from WT mice, IFNγR−/− mice, and CCR2−/− mice inoculated for 24 h with F. tularensis and restimulated with heat-killed F. tularensis in culture. *p < 0.05 WT versus IFNγR−/− or CCR2−/− mice. Data are representative of at least two repeated experiments.
Discussion
In the present study, we describe a host defense circuit activated by F. tularensis whereby MyD88 mediates production of IFNγ, which in turn signals via its receptor in BM-derived cells, resulting in the production of MCP-1 and subsequent CCR2-dependent mobilization of monocyte cells to the spleen. There, mobilized monocytes subsequently produce TNF-α and NO and mediate clearance of F. tularensis.
The role of IFNγR signaling in the activation of innate immune responses is critical to host defense in a wide range of in vivo models of intracellular bacterial infection, including systemic Mycobacterium tuberculosis, and L. monocytogenes infections 6, 33, 34. Likewise, IFNγR signaling is absolutely required for control of F. tularensis infection, and mice lacking IFNγ are rendered moribund 5-7 days after challenge, consistent with our observations in this study 19, 35. IFNγ activates a wide range of antimicrobial mechanisms, including induction of iNOS and subsequent NO production, which is critical for the destruction of pathogenic organisms 36. However, the question of how IFNγ may fit into the wider signaling network that regulates the trafficking of immune cells, particularly myeloid cells, during the innate immune response to infection has not been well characterized. Thus, in the present study, we have uncovered a novel function of IFNγR signaling as a critical regulator of monocyte trafficking in response to systemic F. tularensis infection.
Our data show that IFNγR-deficient mice have decreased systemic levels of MCP-1, which suggests that induction of MCP-1 is a mechanism by which IFNγ orchestrates the mobilization of monocytes. We confirmed this mechanism by showing that mice deficient in CCR2, the MCP-1 receptor, also fail to mobilize monocytes to the spleen in response to F. tularensis challenge. Analysis of the MCP-1 promoter and enhancer has uncovered the presence of NF-κB and STAT-1 binding sites. In vitro studies have shown that MCP-1 is induced by a number of NF-κB-activating inflammatory signals, including TNF-α and TLR signaling in a wide range of cell types, including myeloid cells 26, 37. F. tularensis, like many other bacteria, contains a complex set of molecular motifs that can induce NF-κB activation via TLRs 16, 38, 39. We were therefore surprised that signaling via TLRs and other inflammatory cytokines activated by F. tularensis infection was not itself sufficient to drive MCP-1 production in IFNγR-deficient mice. However, our in vitro experiments suggest that IFNγ synergistically enhances otherwise modest F. tularensis-induced MCP-1 production, suggesting that F. tularensis alone may not efficiently trigger an immune response, and thus IFNγ functions as an amplifying loop, resulting in increased MCP-1 production and efficient mobilization of monocytes. Interestingly, F. tularensis LPS has been shown to be a weak activator of TLR signaling in vitro and in vivo 40, 41, 42, 43. Furthermore, F. tularensis has been shown to impair the production of inflammatory cytokines in infected cells 44. Thus, IFNγR signaling may serve to counteract these unique properties of F. tularensis, which may serve as possible immune evasion mechanisms, allowing the activation of a robust host defense response to F. tularensis. Further studies should directly compare how efficiently different intracellular bacteria, including F. tularensis, or their components activate immune responses in monocytes and macrophages.
Our data demonstrate a role for MyD88-dependent signaling in the induction of IFNγ and subsequent MCP-1 production. These findings are consistent with previous work showing defective IFNγ production in F. tularensis-infected MyD88-deficient mice 29. MyD88 functions downstream of three major host defense signaling mechanisms, TLRs, IL-1R, and IL-18R 30. Prior studies have suggested a role for TLR2 in the recognition of F. tularensis lipoproteins, and while we have not observed decreased IFNγ production in TLR2-deficient mice (our unpublished observation), it is unclear whether TLR2 is the only TLR to recognize F. tularensis 16, 45. Interestingly, F. tularensis-infected IL-18R-deficient mice produce less IFNγ (our unpublished observation), suggesting that signaling downstream of IL-18R may be part of the MyD88-dependent host defense mechanism described in our study. IL-18, in conjunction with IL-12, activates IFNγ production by NK cells and T lymphocytes 46. Both NK and T cell populations produce IFNγ in response to F. tularensis, suggesting that NK cells and T cells may be critical sources of IFNγ during the host response against F. tularensis infection 23, 35, 47. Interestingly, work using Rag1-deficient mice suggests that early IFNγ production can occur independently of T lymphocytes, as the proportion of IFNγ-producing cells is not decreased in these mice 48. Conversely, mice depleted of NK cells have lower proportions of IFNγ-producing cells three days after F. tularensis inoculation 23, 47. Notably, one study demonstrated that the efficient formation of myeloid granulomas in the liver after F. tularensis infection required both IFNγ and NK cells, linking NK cell-mediated IFNγ production to myeloid cell trafficking 23. While these findings suggest that NK cells in particular may be largely responsible for early IFNγ production, depletion of NK cells in mice prior to infection did not increase their susceptibility to F. tularensis challenge, suggesting that other cell types may produce enough IFNγ to sufficiently activate host defense 47, 49. Interestingly, IFNγ expression in response to F. tularensis infection has been detected in dendritic cells and neutrophils, as well as hybrid NKDC populations, suggesting that IFNγ production by these non-lymphoid cell types may contribute to early activation of host defense against F. tularensis in the absence of T or NK cells 48. Based on these studies, we speculate that MyD88-dependent IL-18R signaling, predominantly in NK and T cells, is a likely mechanism by which IFNγ production is activated in response to F. tularensis. Furthermore, the observation that myeloid populations can produce IFNγ in response to F. tularensis raises the interesting possibility that IL-18R/MyD88-dependent IFNγ production by these cells could contribute to the induction of MCP-1 expression.
We found that CCR2-deficient mice failed to mobilize monocytes to the spleen after F. tularensis challenge, and have significantly elevated bacterial numbers in the spleen compared to WT mice. These observations serve as direct evidence that monocytes are critical for efficient control of F. tularensis infection. CCR2-deficient mice serve as a compelling and widely used model for studying the specific contribution of monocytes to host defense, as these mice fail to mobilize monocytes to the periphery, including the spleen, upon infection or inflammation 7, 31, 32. Importantly, the number and function of other cell types, including lymphocytes, neutrophils, tissue macrophages, and monocytes themselves, are not greatly perturbed by loss of CCR2 signaling at the steady state, making use of this model particularly advantageous 7, 31, 32. The defects in host defense we have observed using CCR2-deficient mice to study the role of monocytes in response to F. tularensis infection are consistent with published studies using CCR2-deficient mice, as well as mice deficient in MCP-1 and other individual CCR2 ligands, to probe the role of monocytes in response to L. monocytogenes infection 5, 7, 50. Collectively, these findings suggest that monocytes are critical mediators of the innate immune response against intracellular bacterial pathogens in general.
Monocytes are thought to control infection by intracellular bacteria in part via production of TNF-α, which activates immune responses via autocrine and paracrine mechanisms; they also activate direct antimicrobial effector mechanisms such as production of NO 5, 10. Both mechanisms have been shown to be critical for clearance of F. tularensis 51, 52. We have shown that production of TNF-α and NO is defective in IFNγR- and CCR2-deficient mice, which at least in part explains the elevated F. tularensis CFU we found in the spleens of these mice. Notably, we found that IFNγR-deficient mice had nearly a log more F. tularensis in their spleens than CCR2-deficient mice, suggesting that IFNγ activates a wider set of antimicrobial mechanisms, in which monocytes play a critical part.
Collectively, our findings describe a novel role for MyD88-dependent IFNγR signaling in the activation of MCP-1 production, leading to mobilization and activation of monocytes, which in turn mediate efficient host defense in part via TNF-α and NO production. Notably, recent work has uncovered a possible role for IFNγ in the activation of hematopoietic stem and progenitor cell proliferation in vivo and in vitro 53. These findings suggest that IFNγ may trigger the de novo generation of myeloid cells to replenish the immune system in addition to inducing MCP-1-dependent mobilization of extant myeloid populations from the BM to the periphery. Furthermore, CCR2 has been shown to regulate the migration of hematopoietic progenitor cells from the BM to the periphery in response to inflammation 54. Combining these findings with our results, the IFNγR/CCR2 signaling axis is a critical mechanism for the activation and remodeling of both immature and mature BM and peripheral hematopoietic compartments in response to inflammation and intracellular pathogen infection.
Materials and Methods
F. tularensis preparation
The F. tularensis live vaccine strain (LVS) was generously provided by Dr Karen Elkins. F. tularensis from frozen aliquots was streaked onto rabbit cysteine blood agar plates (Remel, Lenexa, KS) and individual colonies of F. tularensis were grown overnight in Mueller-Hinton Broth (MHB) supplemented with 1% Isovitalex (BD Biosciences), 0.1% glucose and ferric pyrophosphate (Sigma-Aldrich). Bacteria were harvested while in mid-log phase as determined by optical density at 600 nm wavelength in a spectrophotometer (Beckman-Coulter), washed twice with sterile PBS and diluted with sterile PBS for infection. Bacterial CFUs were verified by plating dilutions onto rabbit cysteine blood agar.
Mice
All procedures described herein were approved by and conducted in compliance with the UCLA Animal Research Committee. 6-8 week-old female C57BL/6J (WT), IFNγR-deficient, IFNαβR-deficient, and CCR2-deficient mice were obtained from the Jackson Laboratories. MyD88-deficient mice and control littermates were obtained from Dr Shizuo Akira. Mice were housed and bred in the UCLA vivarium in specific pathogen-free conditions.
Intravenous F. tularensis systemic infection model
For in vivo infections, mice were inoculated via the lateral tail vein with 2 × 104 CFU F. tularensis grown to log-phase and prepared as described above in a 200-μl bolus using a 28-gauge syringe (Becton-Dickenson), and euthanized 24 h after challenge. Groups of three mice were used in each experiment. For quantification of F. tularensis CFUs, spleens were homogenized to single-cell suspension and serially plated on rabbit cysteine blood agar plates for colony counting.
Generation of BM chimeras
BM reconstitution experiments were performed as previously described 55. Whole BM was flushed from the femurs and tibiae of donor mice and depleted of RBCs using ACK lysis buffer (0.15 M ammonium chloride (NH4Cl), 1 mM potassium bicarbonate (KHCO3), and 0.1 mM EDTA (pH 7.3)). BM cells were subsequently washed twice in PBS and counted on a hemocytometer. WT recipient mice were lethally irradiated with 1100 Rad and reconstituted 24 h later by injection of 1 × 107 WT or IFNγR-deficient donor BM cells via the lateral tail vein. Mice were maintained in autoclaved cages, given irradiated feed and were maintained on sulfamethoxazole and trimethoprim oral suspension (TMS; 48 mg/ml in drinking water) for three weeks after irradiation. Mice were used for experimentation 8 weeks after BM reconstitution.
Flow cytometry
Spleens were homogenized into a single-cell suspension and depleted of RBC using ACK lysis buffer. Splenocytes were resuspended in staining media consisting of PBS containing 2% FBS and stained with anti-CD11b-APC or FITC, and anti-Ly6C-FITC or anti-Ly6C-biotin on ice for 30 min. All antibodies and SA-APC fluorochrome were obtained from BD Pharmingen (San Jose, CA). Cells were then washed and fixed with 2% PFA prior to analysis on a FACScalibur flow cytometer. For intracellular stains, 2 × 105 splenocytes were resuspended in DMEM containing 10% FBS containing 1:1 000 BD GolgiPlug and 2 × 106 heat-killed F. tularensis for 4 h in a 37 °C humidified incubator. Cells were subsequently stained for CD11b, fixed and permeabilized using BD Cytofix/Cytoperm, and stained for intracellular TNF-α using an anti-TNF-α-PE antibody for 30 min on ice. Cells were washed with BD PermWash and analyzed on a BD FACSCalibur. All flow cytometry data were analyzed using FlowJo (TreeStar, Inc., Palo Alto, CA, USA).
In vitro F. tularensis infection
WT BMMs were generated from whole mouse BM as previously described 56. For in vitro infections, bacteria were diluted in DMEM and added at an MOI of 1 to 1 × 106 BM macrophages (for Q-PCR analysis) or 2 × 104 BM macrophages (for ELISA analysis) cultured in antibiotic-free media containing 5% heat-inactivated FBS. Bacteria were spun onto cells at 2 000 rpm for 10 min in a Beckman centrifuge at room temperature. Media was washed off 1 h later and replaced with media containing 2 μg/ml gentamicin (Sigma-Aldrich). Pam3Cys (Sigma-Aldrich) was added at 0.01 ng/ml; recombinant murine IFNγ (R&D Systems) was added at concentrations ranging from 0.01 to 10 ng/ml. For MCP1 mRNA expression analysis experiments, cells were washed with PBS 6 h later and harvested in 1 ml of Trizol (Invitrogen). For MCP-1 ELISA, supernatants were recovered 24 h later.
Cytokine expression analysis by quantitative RT-PCR
Total RNA was harvested from BMMs or washed splenocytes using Trizol (Invitrogen) according to the manufacturer's instructions. cDNA was generated from RNA using iScript (Bio-Rad). Quantitative RT-PCR analysis of MCP-1 mRNA levels was carried out on an iCycler (Bio-Rad) using Sybr Green 2× Master Mix (Applied Biosystems). Primer sequences used are MCP1 Fwd 5′-GCTGACCCCAAGAAGGAATG-3′, Rev 5′-GAAGACCTTAGGGCAGATGCA-3′. Data were normalized to L32 mRNA levels.
ELISAs
ELISA development kits for MCP-1 (eBioscience, San Diego, CA, USA) were used according to the manufacturer's instructions and analyzed using a 96-well plate spectrophotometer (Thermo-Fisher, Waltham, MA, USA). Spleens to be analyzed for cytokine levels by ELISA were weighed and homogenized in 0.5 ml PBS containing 0.05% Triton X-100 and centrifuged to remove debris. Supernatants were collected from 96-well plates and centrifuged to remove debris. All samples were plated in duplicate. Cytokine concentrations obtained from spleen homogenates were normalized to spleen mass.
Statistical analysis
The unpaired Student's t-test was used to compare groups; P-values of 0.05 or less were considered to be statistically significant.
References
Fearon DT, Locksley RM . The instructive role of innate immunity in the acquired immune response. Science 1996; 272:50–53.
Takeda K, Akira S . Toll-like receptors in innate immunity. Int Immunol 2005; 17:1–14.
Pedra JH, Cassel SL, Sutterwala FS . Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol 2009; 21:10–16.
Fritz JH, Girardin SE . How Toll-like receptors and Nod-like receptors contribute to innate immunity in mammals. J Endotoxin Res 2005; 11:390–394.
Serbina NV, Jia T, Hohl TM, Pamer EG . Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 2008; 26:421–452.
Busch DH, Vijh S, Pamer EG . Animal model for infection with Listeria monocytogenes. Curr Protoc Immunol 2001; Chapter 19:Unit 19.9.
Serbina NV, Pamer EG . Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 2006; 7:311–317.
Huffnagle GB, Traynor TR, McDonald RA, et al. Leukocyte recruitment during pulmonary Cryptococcus neoformans infection. Immunopharmacology 2000; 48:231–236.
Amano H, Morimoto K, Senba M, et al. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol 2004; 172:398–409.
Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG . TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003; 19:59–70.
Boehm U, Klamp T, Groot M, Howard JC . Cellular responses to interferon-gamma. Annu Rev Immunol 1997; 15:749–795.
Leite-de-Moraes MC, Dy M . Natural killer T cells: a potent cytokine-producing cell population. Eur Cytokine Netw 1997; 8:229–237.
Schoenborn JR, Wilson CB . Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 2007; 96:41–101.
Kleinert H, Schwarz PM, Forstermann U . Regulation of the expression of inducible nitric oxide synthase. Biol Chem 2003; 384:1343–1364.
Shtrichman R, Samuel CE . The role of gamma interferon in antimicrobial immunity. Curr Opin Microbiol 2001; 4:251–259.
Cole LE, Shirey KA, Barry E, et al. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect Immun 2007; 75:4127–4137.
Collazo CM, Sher A, Meierovics AI, Elkins KL . Myeloid differentiation factor-88 (MyD88) is essential for control of primary in vivo Francisella tularensis LVS infection, but not for control of intramacrophage bacterial replication. Microbes Infect 2006; 8:779–790.
Sjostedt A, Conlan JW, North RJ . Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun 1994; 62:2779–2783.
Anthony LS, Ghadirian E, Nestel FP, Kongshavn PA . The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog 1989; 7:421–428.
Anthony LS, Morrissey PJ, Nano FE . Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from L-arginine metabolism. J Immunol 1992; 148:1829–1834.
Elkins KL, Colombini SM, Meierovics AI, Chu MC, Chou AY, Cowley SC . Survival of secondary lethal systemic Francisella LVS challenge depends largely on interferon gamma. Microbes Infect 2010; 12:28–36.
Sjostedt A, North RJ, Conlan JW . The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 1996; 142 (Pt 6):1369–1374.
Bokhari SM, Kim KJ, Pinson DM, Slusser J, Yeh HW, Parmely MJ . NK cells and gamma interferon coordinate the formation and function of hepatic granulomas in mice infected with the Francisella tularensis live vaccine strain. Infect Immun 2008; 76:1379–1389.
Billiau A, Matthys P . Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev 2009; 20:97–113.
Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA . Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun 1991; 59:2922–2928.
Ping D, Jones PL, Boss JM . TNF regulates the in vivo occupancy of both distal and proximal regulatory regions of the MCP-1/JE gene. Immunity 1996; 4:455–469.
Satriano JA, Hora K, Shan Z, Stanley ER, Mori T, Schlondorff D . Regulation of monocyte chemoattractant protein-1 and macrophage colony-stimulating factor-1 by IFN-gamma, tumor necrosis factor-alpha, IgG aggregates, and cAMP in mouse mesangial cells. J Immunol 1993; 150:1971–1978.
Martin CA, Dorf ME . Differential regulation of interleukin-6, macrophage inflammatory protein-1, and JE/MCP-1 cytokine expression in macrophage cell lines. Cell Immunol 1991; 135:245–258.
Collazo CM, Sher A, Meierovics AI, Elkins KL . Myeloid differentiation factor-88 (MyD88) is essential for control of primary in vivo Francisella tularensis LVS infection, but not for control of intra-macrophage bacterial replication. Microbes Infect 2006; 8:779–790.
Medzhitov R, Preston-Hurlburt P, Kopp E, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 1998; 2:253–258.
Kurihara T, Warr G, Loy J, Bravo R . Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 1997; 186:1757–1762.
Boring L, Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 1997; 100:2552–2561.
Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR . An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993; 178:2249–2254.
Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG . TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003; 19:59–70.
Elkins KL, Rhinehart-Jones TR, Culkin SJ, Yee D, Winegar RK . Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun 1996; 64:3288–3293.
Karupiah G, Hunt NH, King NJ, Chaudhri G . NADPH oxidase, Nramp1 and nitric oxide synthase 2 in the host antimicrobial response. Rev Immunogenet 2000; 2:387–415.
Ping D, Boekhoudt GH, Rogers EM, Boss JM . Nuclear factor-kappa B p65 mediates the assembly and activation of the TNF-responsive element of the murine monocyte chemoattractant-1 gene. J Immunol 1999; 162:727–734.
Kovarova H, Marcela A, Stulik J . Macrophage activating factors produced in the course of murine tularemia: effect on multiplication of microbes. Arch Immunol Ther Exp (Warsz) 1992; 40:183–190.
Cole LE, Santiago A, Barry E, et al. Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways. J Immunol 2008; 180:6885–6891.
Vinogradov E, Perry MB, Conlan JW . Structural analysis of Francisella tularensis lipopolysaccharide. Eur J Biochem 2002; 269:6112–6118.
Dreisbach VC, Cowley S, Elkins KL . Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun 2000; 68:1988–1996.
Duenas AI, Aceves M, Orduna A, et al. Francisella tularensis LPS induces the production of cytokines in human monocytes and signals via Toll-like receptor 4 with much lower potency than E. coli LPS. Int Immunol 2006; 18:785–795.
Hajjar AM, Harvey MD, Shaffer SA, et al. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun 2006; 74:6730–6738.
Telepnev M, Golovliov I, Grundstrom T, Tarnvik A, Sjostedt A . Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-alpha and IL-1 from murine macrophages. Cell Microbiol 2003; 5:41–51.
Malik M, Bakshi CS, Sahay B, Shah A, Lotz SA, Sellati TJ . Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun 2006; 74:3657–3662.
Dinarello CA . Interleukin-18. Methods 1999; 19:121–132.
Lopez MC, Duckett NS, Baron SD, Metzger DW . Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell Immunol 2004; 232:75–85.
De Pascalis R, Taylor BC, Elkins KL . Diverse myeloid and lymphoid cell subpopulations produce gamma interferon during early innate immune responses to Francisella tularensis live vaccine strain. Infect Immun 2008; 76:4311–4321.
Leiby DA, Fortier AH, Crawford RM, Schreiber RD, Nacy CA . In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun 1992; 60:84–89.
Jia T, Serbina NV, Brandl K, et al. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol 2008; 180:6846–6853.
Lindgren H, Stenman L, Tarnvik A, Sjostedt A . The contribution of reactive nitrogen and oxygen species to the killing of Francisella tularensis LVS by murine macrophages. Microbes Infect 2005; 7:467–475.
Chen W, KuoLee R, Shen H, Conlan JW . Susceptibility of immunodeficient mice to aerosol and systemic infection with virulent strains of Francisella tularensis. Microb Pathog 2004; 36:311–318.
Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA . Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature 2010; 465:793–797.
Si Y, Tsou CL, Croft K, Charo IF . CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest 2010; 120:1192–1203.
Miller LS, Pietras EM, Uricchio LH, et al. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol 2007; 179:6933–6942.
Doyle SE, O'Connell RM, Miranda GA, et al. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med 2004; 199:81–90.
Acknowledgements
We would like to thank Dr Karen Elkins (US Food and Drug Administration (FDA), USA) for kindly providing the F. tularensis LVS. We also thank Dr Shizuo Akira (Osaka University, Japan) for the generous gift of MyD88-deficient mice. This work was supported by grants R01 AI056154, R01 CA87924, and R01 AI052359 (to GC). This work was also supported by the Ruth L Kirschstein Research Service Award GM 007185 (to EMP).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Defective host defense in IFNγR-deficient mice in response to systemic F. tularensis challenge. (PDF 245 kb)
Supplementary information, Figure S2
Defective host defense and bacterial clearance in MyD88-deficient mice during systemic F. tularensis challenge. (PDF 264 kb)
Rights and permissions
About this article
Cite this article
Pietras, E., Miller, L., Johnson, C. et al. A MyD88-dependent IFNγR-CCR2 signaling circuit is required for mobilization of monocytes and host defense against systemic bacterial challenge. Cell Res 21, 1068–1079 (2011). https://doi.org/10.1038/cr.2011.59
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2011.59
Keywords
This article is cited by
-
Innate immune responses at the asymptomatic stage of influenza A viral infections of Streptococcus pneumoniae colonized and non-colonized mice
Scientific Reports (2021)
-
Opposing effects of antibiotics and germ-free status on neuropeptide systems involved in social behaviour and pain regulation
BMC Neuroscience (2020)
-
CCR2 mediates increased susceptibility to post-H1N1 bacterial pneumonia by limiting dendritic cell induction of IL-17
Mucosal Immunology (2019)