Abstract
To understand the molecular mechanisms underlying chloroplast development, we isolated and characterized the albino mutant emb1303-1 in Arabidopsis. The mutant displayed a severe dwarf phenotype with small albino rosette leaves and short roots on a synthetic medium containing sucrose. It is pigment-deficient and seedling lethal when grown in soil. Embryo development was delayed in the mutant, although seed germination was not significantly impaired. The plastids of emb1303-1 were arrested in early developmental stages without the classical stack of thylakoid membrane. Genetic and molecular analyses uncovered that the EMB1303 gene encodes a novel chloroplast-localized protein. Microarray and RT-PCR analyses revealed that a number of nuclear- and plastid-encoded genes involved in photosynthesis and chloroplast biogenesis were substantially downregulated in the mutant. Moreover, the accumulation of several major chloroplast proteins was severely compromised in emb1303-1. These results suggest that EMB1303 is essential for chloroplast development.
Similar content being viewed by others
Introduction
Chloroplast is a special and important organelle of plant cells carrying out many essential processes such as photosynthesis and biosynthesis of fatty acids, pigments, and amino acids from inorganic nitrogen 1.
In addition to the outer and inner membrane, mature chloroplasts have an internal membrane network of thylakoids, where the light reactions take place, converting the light energy into chemical energy stored in ATP and NADPH. The formation of the chloroplast complex and elaborate thylakoid membrane initiates from proplastids and is dependent on the coordinated expression of nuclear- and plastid-encoded genes 2, 3. These genes encode proteins involved in many processes of chloroplast development such as RNA processing, protein translation and folding, and protein transport. Such proteins include PAC, a nuclear-encoded protein functioning in plastid mRNA maturation and accumulation 4; HCF136, a chaperone-like assembly factor for the stability of photosystem II (PSII) 5; SLP, chaperonin-60α for protein folding 6; APG2, a major component of ΔpH-dependent thylakoid protein transporter 7; and ALB3, a subunit of the thylakoid Sec protein transport system 8, 9. Loss of these proteins usually impairs chloroplast development resulting in abnormal chloroplast morphology. Chloroplast is also involved in the biosynthesis of plastid isoprenoids such as chlorophyll and carotenoid. Mutations in the isoprenoid biosynthesis pathways, such as cla1, ispD, ispE, ispG, ispH, pds3, and zds, block chloroplast development and result in albino phenotype 10, 11, 12, 13, 14.
Chloroplast development is tightly linked to embryogenesis. Mutants interfering with or blocking chloroplast development usually exhibit embryogenesis defects 6, 15, 16, 17, 18, 19. For example, mutations in some genes participating in chloroplast membrane lipid biosynthesis result in embryonic lethality 20, 21. However, a deficiency in the photosynthetic capacity of embryo plastids does not always cause an embryonic lethal phenotype. Some albino and pigment-deficient mutants produce morphologically normal seeds that are able to germinate and grow to variable extent in the presence of sugar 22. Similarly, the majority of mutations in genes involved in photosynthesis, such as VAR2, PsbP, and CHL27, do not confer embryonic lethality, but produce pale green to yellow or variegated seedlings 1, 23, 24. Mutations in genes in plastid isoprenoid biosynthesis pathways, such as CLA1, PDS3, and ZDS, do not show embryonic lethal phenotype either 10, 13, 14.
Despite the discovery of many genes involved in chloroplast development, we still do not have a full understanding of the complex biogenesis of this organelle. In this study, we isolated an albino mutant emb1303-1, which is defective in a gene coding for a new chloroplast protein. Physiological and molecular analyses suggest that EMB1303 plays an essential role in both chloroplast development and plant growth.
Results
Isolation and phenotypic characterization of an albino mutant
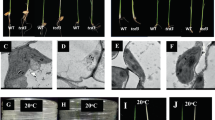
The albino mutant was isolated by screening Arabidopsis T-DNA insertion lines that had been mutagenized by T-DNA insertion (ABRC (Arabidopsis Biological Resource Center)). The homozygous mutant plants showed albino phenotype and died shortly after germination in soil. On Murashige-Skoog (MS) medium supplemented with 2% sucrose, the mutant plants germinated normally and had purple cotyledons that became white several days later (Figure 1A). The growth of the mutant was arrested in subsequent development with smaller rosette leaves and shorter roots compared to the Col-0 wild type (Figure 1B and 1C). The mutant had swollen white leaves with curved and abnormal edges. Light microscopy analysis showed that the mutant leaves had a reduced number of mesophyll cells with large intercellular spaces and lacked defined mesophyll layers (Figure 1D and 1E). The pavement cells in the mutant leaf epidermis did not have the interlocking jigsaw puzzle-shape but were rather rectangular or spindle-like (Figure 1F and 1G). Scanning electron microscopy analysis showed that pavement cells at both abaxial and adaxial surfaces were abnormal in the mutant, and the lobes of the cell were dramatically eliminated compared to the wild type (Figure 2).
Growth phenotypes of the emb1303 mutants. Wild-type and the emb1303 mutant seeds were grown at 22 °C under long-light conditions for 5 days (A), and 35 days (C). (B) Leaves and inflorescences of wild type (top) and emb1303-1 (bottom). (D, E) Cross-sections of leaves from the wild type (D) and emb1303-1 (E). Bar: 100 μm. (F, G) Pavement cells from true leaves of wild type (F) and emb1303-1 (G) stained by propidium iodide. Bar: 100 μm.
The primary root of the mutant was much shorter than that of the wild type (Figure 1C). When grown on the medium containing norflurazon that blocks the synthesis of chlorophyll and carotenoid, the mutant still had shorter primary root compared to the wild type (data not shown). This suggests that the impaired root development is partially independent of the chlorophyll and carotenoid levels. The mutant could bolt eventually in tissue culture with sucrose, but the flowers were infertile.
Molecular characterization and complementation of emb1303
To investigate the nature of the mutation, we analyzed the progeny of a backcross between the mutant and the wild-type Col-0. All of the F1 progeny (n = 18) showed a normal phenotype, indicating that the mutation was recessive. Among the 350 F2 progeny, the mutant phenotype segregated at a 1:3 ratio (mutant:WT = 88:270, χ2 = 0.059, P < 0.05). Further analysis showed that all the 88 albino plants were kanamycin-resistant (Kan-R). We also randomly chose 200 Kan-R individuals from the F2 and found that the albino phenotype segregated at a 1:2 ratio (mutant:WT = 64:136, χ2 = 0.202, P < 0.05). These results suggest that the albino phenotype is tightly linked to a T-DNA insertion at a single locus.
The flanking sequences of the T-DNA insertion were amplified by thermal asymmetric interlaced PCR. Two left borders of the T-DNA insertion were found to be located in the upstream and downstream of At1g56200 (Figure 3A). Further PCR analysis revealed that the genomic fragment of At1g56200 was deleted in the mutant. At1g56200 was previously annotated as Embryo Defective 1303 (EMB1303), and we therefore named this mutant as emb1303-1 hereafter.
Isolation of the emb1303 mutants. (A) A schematic diagram of genomic structure of the EMB1303 gene. Filled boxes and lines indicate exons and introns, respectively. Positions of T-DNA insertions are indicated by triangles with left border indicated by arrows. The T-DNA insertion site in emb1303-1 spans EMB1303 gene, leading to a deletion of the gene. The T-DNA in emb1303-2 is located at 5′-UTR of EMB1303. (B) RT-PCR analysis of EMB1303 transcripts in the wild type and emb1303 mutants. β-tubulin gene TUB8 was used as a control. (C) Complementation of the emb1303-1 mutant with genomic fragment of EMB1303 gene.
To confirm that the disruption of EMB1303 is responsible for the albino phenotype, we cloned a 5.2-kb wild-type genomic fragment of EMB1303 from the BAC clone F14G9, and transformed it into heterozygous emb1303-1 plants. Among 48 T1 transgenic lines analyzed, 13 lines were homozygous emb1303-1 and they showed a wild-type looking phenotype (Figure 3C). We also identified a second mutant allele emb1303-2 (CS16150) in Ws background from ABRC. emb1303-2 carried a T-DNA insertion at 5′-UTR of At1g56200 (Figure 3A), and exhibited a similar phenotype to emb1303-1 (Figure 1C). RT-PCR analysis showed that the T-DNA insertions abolished EMB1303 mRNA expression in both alleles of emb1303 (Figure 3B), indicating that they are loss-of-function mutants. Collectively, these results demonstrate that EMB1303 is indeed At1g56200. Since these two alleles showed similar phenotypes, we chose emb1303-1 for further studies unless specified otherwise.
Molecular characterization of EMB1303
There are two splicing variants of EMB1303 cDNA with four exons and three introns each in Arabidopsis, thus encoding two putative proteins with 151 and 154 amino acids, respectively. A BLAST search revealed that the predicted EMB1303 protein and its homolog (At1g30475) in Arabidopsis share 57% identity and 71% similarity at the amino acid level (Supplementary information, Figure S1). EMB1303 also shares significant identity with the rice proteins LOC_Os02g17380, LOC_Os02g05890, LOC_Os06g22660, Megicago truncatula protein ACJ85874, and rape protein CAO45180, with high degrees of conservation at the carboxyl-terminus. However, these proteins do not have known domains or motifs predicted by any available bioinformatics tools, and no functional data are available in plants.
In order to elucidate the physiological function of EMB1303, we examined its organ-specific expression in Arabidopsis. EMB1303 was strongly expressed in young leaves, roots, and flowers (Figure 4A). This result was validated by β-glucuronidase (GUS) staining on transgenic plants expressing GUS driven by the EMB1303 promoter. The GUS activity was high in cotyledons and young rosette leaves, but low in stems (Figure 4B-4E). GUS expression was also detected in the open anthers, style and ovary walls, and young siliques, whereas it was not detected in young flower buds, immature pollen, or mature siliques (Figure 4F-4H). These results suggest that EMB1303 may function in a number of developmental stages.
Spacial expression pattern of the EMB1303 gene. Spacial expression pattern of the EMB1303 gene. (A) RT-PCR analysis of EMB1303 transcripts in various organs of Arabidopsis plants. Total RNA was isolated from the following tissues: roots (R), stems (S), leaves (L), young flowers (YF), maturing flowers (MF), and mature siliques (SL) of Col plants grown under long-day conditions. RT-PCR was performed with EMB1303-specific primers (top gel) and TUB8-specific primers (bottom gel). (B-H) GUS expression in seedlings at the cotyledon (B), four-leaf (C) and eight-leaf (D) stages, stem and cauline leaf (E), inflorescence and flowers (F, G), and mature silique (H) of pEMB1303::GUS transgenic plants.
We further generated transgenic plants overexpressing EMB1303 under the control of CaMV 35S promoter. However, none of the transgenic plants showed visible growth defect phenotypes (data not shown), indicating that constitutive expression of EMB1303 does not significantly affect growth and development.
Localization of the EMB1303 protein
P-Sort and Target P analyses predict that EMB1303 is likely targeted to the chloroplast. Computational transit peptide prediction 25 also reveals the presence of a probable chloroplast-targeting sequence at the N-terminal region of EMB1303.
To determine the subcellular localization of EMB1303, we generated a construct p35S::EMB1303-GFP containing genomic fragment of EMB1303 fused in-frame to a synthetic green fluorescent protein at the C-terminal region. Transient expression of this fusion protein in Arabidopsis protoplasts and Nicotiana benthamiana leaves was examined by confocal microscopy. The green fluorescence was co-localized with the red autofluorescence of chlorophyll in leaf mesophyll cells of N. benthamiana (Figure 5A). The control protein expressed from p35S::GFP was localized ubiquitously. Further analysis showed that GFP signals were in the chloroplasts of the protoplasts isolated from leaves from N. benthamiana harboring p35S::EMB1303-GFP (Figure 5B). After these protoplasts were broken, the GFP fluorescence was still co-localized with the chloroplasts from p35S::EMB1303-GFP protoplasts, while the GFP signal was nearly abolished with the removal of the cytoplasm of p35S::GFP protoplasts, indicating that EMB1303-GFP is targeted to the chloroplast (Figure 5C). Similar results were obtained with transiently expressed p35S::EMB1303-GFP in Arabidopsis protoplasts (data not shown).
Subcellular localization of the EMB1303 protein in N. bethamiana leaves. p35S::EMB1303-GFP and p35S::GFP were transformed into N. benthamiana by A. tumefaciens-mediated infiltration. Signals were detected by a laser confocal-scanning microscope from intact leaf mesophyll cells (A). (B) Protoplasts prepared from the same leaves as in (A) , and (C) broken protoplasts from (B). Green fluorescence signals, chlorophyll red autofluorescence, an overlay of green and red signals, and bright-field images are shown in panels (in the order from left to right). Bar: 20 μm. (D) Immunoblot assay of proteins. Total proteins (T) and chloroplast proteins (Chl) were extracted from protoplasts prepared from the same leaves of (A) and analyzed by immunoblotting with polyclonal antibodies raised against GFP.
In addition, chloroplasts were isolated from protoplasts of N. benthamiana leaves harboring p35S::EMB1303-GFP, and the total proteins were subject to immunoblot analysis with an antibody against GFP protein. EMB1303-GFP protein was detected in chloroplast fraction with a molecular weight at approximately 37 kDa, which is smaller than the predicted molecular mass of 43 kDa for the full-length protein (Figure 5D). It is however consistent with the mass of the protein where the N-terminal signal sequence is removed after importing into the chloroplast. Taken together, these results indicate that EMB1303 is localized in the chloroplast.
Seedling morphology of emb1303-1
It is known that under relatively high light conditions, photo-oxidative stress occurs in the mutants defective in carotenoid biosynthesis, resulting in abnormal chloroplast development 26. To determine whether the emb1303-1 phenotype is caused by photo-oxidative stress or merely due to abnormal plastid development, we grew emb1303-1 under different light conditions. Under high light, the growth of emb1303-1 seedlings was arrested. A typical 14-day-old wild-type seedling had four true leaves and long primary root with multiple lateral shoots, whereas emb1303-1 had only two small true leaves and one short primary root (Supplementary information, Figure S2A). The mutant showed slightly better growth under low light or darkness than under high light, but the albino phenotype could not be rescued (Supplementary information, Figure S2B-2D). This result indicates that the albino phenotype of emb1303-1 largely results from a block in plastid development, but not from the photo-oxidative damage caused by deficiency in photo-protective carotenoids.
It has been reported that glucose and sucrose regulate expression of genes involved in accumulation, mobilization, and storage of photosynthetic products 27, thus modulating plant growth and development. To investigate the effect of sugar on the growth of emb1303-1, we grew emb1303-1 on MS media with or without sucrose. The development of emb1303-1 was completely arrested after germination under sucrose-depleted condition, resembling growth in soil (Supplementary information, Figure S2E). In contrast, emb1303-1 showed better growth on sucrose-containing medium (Figure 1B, Supplementary information, Figure S2F-2G). Besides, emb1303-1 seedlings accumulated more anthocyanin under high sugar conditions than the wild type (data not shown), presumably due to the osmotic stress caused by sugar. These results demonstrate that the mutant is not able to grow photoautotrophically.
Embryogenesis in emb1303-1
A previous study reported that chloroplasts are formed transiently and transformed into storage organelles during early embryo development 28. Since emb1303-1 showed the albino phenotype, we speculate that embryogenesis in self-fertilizing heterozygous emb1303-1 might be abnormal due to defective chloroplasts in the embryo. Within the immature siliques of the self-fertilizing heterozygous emb1303-1, abnormal white seeds were detected at a frequency of about 25% (Figure 6B, 6C) at early stages of embryogenesis. However, the seed color was indistinguishable between the wild type and the mutant after seed desiccation (Figure 6D), indicating a normal development of seed coat in emb1303-1.
Phenotypes of emb1303-1 embryos during seed development. (A) Wild-type seeds. (B-D) Seed segregation in siliques from an emb1301/+ heterozygous plant at different stages. Approximately, one-quarter of albino embryos segregated at early stages. (E-P) Cleared seeds observed under Nomarski optics. Embryo development of wild type (E, G, I, K, M, O) and emb1303-1 (F, H, J, L, N, P) at globular, heart, torpedo and early cotyledon, and mature cotyledon stages. Bar: 100 μm.
Embryo development was further examined by Nomarski microscopy (Figure 6E-6N). It was difficult to distinguish the mutant seeds from the wild-type ones in the heterozygous siliques before the embryos became green. At the greening stage, albino white seeds were observed at a frequency of approximately 25% (white seeds:green seeds = 68:200). Moreover, development of the albino homozygous embryos was retarded. While the embryos of wild-type or heterozygous seeds (n = 158) have already developed into heart-shaped embryos (Figure 6E, 6G), the emb1303-1 embryos (n = 54, equivalent to 25% of the total number 212) were still at the globular stage (Figure 6F, 6H). As green embryos of wild-type or heterozygous seeds (n = 151) progressed to the torpedo or early cotyledon stage (Figure 6I, 6K), embryo development of the homozygous seeds (n = 53) was delayed at the heart stage (Figure 6J, 6L). Nevertheless, tissue organization of the emb1303-1 embryo appeared to be normal at early stages of embryogenesis. The homozygous seeds could form mature embryos eventually, although the size of embryos was small and the color was pale (Figure 6M-6P). These results demonstrate that embryo development of emb1303-1 is delayed during early stages of seed development.
Content of chlorophyll and carotenoid in emb1303-1
It has been shown that many mutants in the methylerythritol 4-phosphate pathway, such as cla1, clb4 (ispG), and clb6 (ispH) in the non-cell-autonomous pathway, contain very low chlorophyll in the seedling, whereas their embryos accumulate significant levels of chlorophyll 29. We examined autofluorescence in the embryos, and found that autofluorescence was much lower in emb1303-1 than in the wild type (Supplementary information, Figure S3A-3B), indicating that EMB1303 acts in a cell-autonomous manner.
We also measured the levels of pigments including chlorophyll and carotenoid in emb1303-1 seedlings. The levels of chlorophyll and carotenoid in emb1303-1 were approximately 1% and 5.7% of those of the wild type, respectively (Table 1). Consistent with this result, the chlorophyll autofluorescence of emb1303-1 was much weaker than that of the wild type (Supplementary information, Figure S3C-3D).
Plastid development in emb1303-1
Loss of photosynthetic pigments may affect chloroplast development. To assess the effect of EMB1303 mutation on chloroplast development, plastids from 28-day-seedling mesophyll cells were examined by transmission electron microscopy. In wild-type plants grown under normal light, chloroplasts were crescent-shaped and contained well-developed thylakoid membranes consisting of stroma thylakoids and grana thylakoids (Figure 7A, 7C). In contrast, plastids in emb1303-1 could not develop into normal mature chloroplasts with the thylakoid membrane system. Instead, all mutant plastids were small, and lacked the lens-like structure typically observed in mature chloroplasts in the wild type (Figure 7B, 7D and 7E). The internal membranes were non-stacked and could not be differentiated into grana and stroma thylakoids. These results suggest that EMB1303 may function in plastid differentiation via mediating internal thylakoid membrane formation. As the morphology of other organelles, such as the endoplasmic reticulum and mitochondria, was similar in the mutant and the wild type (Figure 7C and 7E), EMB1303 mutation may specifically affect chloroplast biogenesis in leaves.
Transmission electron microscopy of plastids from emb1301-1 seedlings. Plants were grown on 1/2 MS medium supplemented with 2% sucrose for 14 days and the second leaf of a representative plant for each phenotype category was fixed for transmission electron microscope analysis. (A, B) An overview of parenchyma cells. Bar: 10 μm. (C, D, E) Enlarged views of chloroplasts. Bar: 500 nm. chl, chloroplast; mit, mitochondrion; st, starch granule.
Expression of genes involved in chlorophyll biosynthesis and chloroplast development in emb1303-1
To elucidate possible effects of the emb1303 mutation on the chlorophyll biosynthesis pathway, we examined the expression of key genes in this pathway, including protochlorophyllide oxidoreductase B (PORB), PORC, chlorophyllide a oxygenase (CAO), and geranylgeranyl reductase (GGRS). Semi-quantitative RT-PCR analysis showed that the levels of PORB, PORC, CAO, and GGRS transcripts were greatly decreased in emb1303-1 (Figure 8A), which likely contributes to the reduced chlorophyll level in the mutant. CLA1 is directly involved in the carotenoid biosynthesis pathway, and its expression was also reduced in the mutant, resulting in the decreased levels of carotenoid.
Gene expression and chloroplast protein expression in emb1303-1 plants. (A) Expression of nuclear- and chloroplast-encoded genes. Total RNA prepared from 3-week-old seedlings of Col and emb1303-1 grown on 1/2 MS media containing 2% sucrose was used for semi-quantitative RT-PCR. The β-tubulin gene (TUB8) was used as a quantitation control. (B) Coomasssie brilliant blue staining of total proteins. Total proteins were extracted from seedlings used in (A) and separated by SDS-PAGE. RBCL refers to the large subunit of Rubisco. (C) Immunoblot analysis of chloroplast proteins. Total proteins were analyzed by immunoblotting with antibodies against D1, D2, LHCII, PsbO, and AtpB proteins.
We further chose nuclear- and chloroplast-encoded genes expressed at different stages of chloroplast development to characterize the plastid differentiation stages in emb1303. The transcript levels of accD 29, 30 and rrn16S 29, marker genes for plastid transcription and translation, respectively, remained the same in the mutant (Figure 8A). This indicates that plastid transcription and translation during the early phases of chloroplast development are not significantly affected by the mutation.
To gain further insight into the role of the EMB1303 gene, we compared the genome-wide expression profile of emb1303-1 seedlings with that of wild-type Col using the Affymetrix ATH1 Genechip (http://www.affymetrix.com/). A total of 1 682 genes were identified with more than threefold change in expression in emb1303-1 as compared with the wild type, with 522 being upregulated and 1 160 downregulated (Supplementary information, Table S1). As expected, the EMB1303 gene was more than 80-fold downregulated in emb1303-1. This Genechip result was validated by RT-PCR experiment. The expression of PORB, PORC, CAO, GGRS, and CLA1 exhibited the same trend in both analyses (Figure 8A).
The differentially expressed genes can be grouped into three major functional categories: metabolism, growth and development, and cell regulation (Supplementary information, Table S1). Interestingly, many genes involved in photosynthesis, chloroplast development, and terpenoid or chlorophyll biosynthesis were drastically downregulated in emb1303-1. Most of them were nuclear-encoded genes, consisting of 14 genes encoding chlorophyll a/b-binding proteins, 19 genes encoding subunits of PSI or II, and 14 genes encoding proteins involved in chlorophyll and carotenoid biosynthesis (Supplementary information, Table S2). It is noteworthy that the expression of chloroplast-encoded genes, including psaA, psaB, psbA, psbB, psbD, psbE, psbG, psbT, ndhG, and rbcL were also reduced in emb1303-1 (Supplementary information, Table S2). Collectively, these results indicate that EMB1303 is required for the expression of genes involved in photosynthesis and chloroplast development.
Immunoblot analysis of chloroplast proteins in emb1303-1
To further investigate chloroplast development in emb1303-1, we analyzed protein profile of emb1303-1. A significant reduction of the large subunit of Rubisco (RBCL) was observed by Coomassie brilliant blue staining (Figure 8B). Immunoblot analyses were also performed with antibodies raised against proteins involved in photosynthesis including D1 and D2 of PSII (encoded by psbA and psbD), the light-harvesting chlorophyll a/b-binding protein (LHCII), a 33-kD protein of the oxygen-evolving complex (PsbO), and the β subunit of the ATP synthase (AtpB). D1 and LHCII were barely detectable in emb1303-1, while D2, PsbO, and AtpB were dramatically reduced (Figure 8C). These results indicate that there are severe defects in the accumulation of photosynthetic protein complexes in emb1303.
Discussion
In this study, we identified a novel albino mutant emb1303 and analyzed the role of the chloroplast-targeted EMB1303 protein in chloroplast development.
Loss of EMB1303 activity results in a complete arrest of plant growth and development and seedling lethality when grown in soil. Although emb1303 mutants could develop through the vegetative stage and even produce inflorescences on the media with sucrose, they are infertile. This shows the necessity of sucrose as an energy source for the mutant growth, indicating that EMB1303 is necessary for the photoautotropic growth of plants.
Though the biochemical activity of EMB1303 is unknown, several lines of evidence suggest that EMB1303 is essential for normal plant growth and chloroplast development. RT-PCR and promoter-reporter analyses have shown that EMB1303 is expressed in almost all tissues and stages. EMB1303-GFP subcellular localization in both N. benthamiana leaves and Arabidopsis protoplasts verifies that EMB1303 is targeted to chloroplast, which was confirmed by immunoblot analysis. Ultrastructure analysis indicates that the plastids of emb1303 are not fully developed and stacked thylakoid membranes are absent in the mutant. Thus, the functional EMB1303 protein is likely required for chloroplast development. Notably, the levels of chlorophyll and carotenoids are dramatically decreased in emb1303, which is consistent with the reduced expression of key genes for chlorophyll and carotenoids biosynthesis, including PORB, PORC, CAO, GGRS, and CLA1. Moreover, chloroplasts of emb1303 are impaired at the early developmental stage, and the accumulation of chloroplast-encoded proteins such as D1 and D2 is significantly affected in emb1303. Consistent with the notion that the impaired chloroplast influences the expression of nuclear genes encoding plastid-localized proteins via retrograde signaling 31, 32, many nuclear genes that are involved in photosynthesis and chloroplast development are downregulated in emb1303 (Supplementary information, Table S2). Alteration of expression of these genes could be responsible for the impaired photosynthetic ability and photoautotrophic growth of emb1303.
In addition to the defect in chloroplast, embryo development of emb1303 is significantly slower than that of the wild type, indicating an important role of EMB1303 in embryogenesis. Our observation is consistent with previous studies showing that albino or pale-green mutants such as edd1, slp, and dcl with defective chloroplast development exhibit abnormal embryogenesis 6, 15, 17. One possibility is that defective embryo development observed in emb1303 is due to photosynthetic dysfunction caused by impairment of thylakoid membranes. It is also possible that the amount of biosynthetic products necessary for embryo development is not enough for the emb1303 embryo to undergo a normal development rate. Further dissection of the biochemical nature of EMB1303 will shed more light on its function in chloroplast development and embryogenesis.
Materials and Methods
Plant material and growth conditions
Arabidopsis thaliana plants were grown at 22 °C under a 16-h light/8-h dark photoperiod at 100 μmol m−2 s−1, with 50-70% relative humidity. Arabidopsis seeds were either directly sown on soil or grown on Petri dishes containing half-strength MS basal salt mixture medium (Sigma) with 2% sucrose and 0.8% agar. N. benthamiana plants were cultivated in greenhouse under a 16-h-light/8-h-dark photoperiod with 50-60% relative humidity at 25 °C.
Two lines with T-DNA insertions in EMB1303, emb1303-1 (SALK_016097) and emb1303-2 (CS16150), were obtained from ABRC. The T-DNA insertion sites were identified by sequencing PCR products amplified from the mutants with T-DNA primers and gene-specific primers EMB1303-1F and EMB1303-1R.
Plasmid construction and plant transformation
Probest High fidelity DNA polymerase (Takara) was used for the amplification reactions and constructs were verified by sequencing.
For the pEMB1303::GUS fusion, a 1.6-kb genomic fragment upstream of the EMB1303 ATG start codon was amplified by PCR using EMB1303-p1F and EMB1303-p1R primers, and it was transcriptionally fused with the GUS reporter gene in the binary vector PZP212 33.
To construct p35S::EMB1303-GFP, a 1.05-kb genomic fragment of EMB1303 was amplified using the primers EMB1303-2F and EMB1303-2R, and cloned into the SmaI and XbaI sites of either pUC-GFP or pGPTVII.GFP 34.
For the molecular complementation assay, a 5.2-kb PstI-XbaI genomic fragment comprising the EMB1303 promoter, the coding region, and the 3′-UTR region isolated from the BAC clone F14G9 from ABRC was cloned into the binary vector pCAMBIA1300 (CAMBIA, Canberra, Australia) to generate the pEMB1303::EMB1303 construct.
Agrobacterium tumefaciens strain GV3101 35 carrying different constructs was used to transform wild-type Col or heterozygous emb1303 mutant plants via floral dip transformation 36. A. tumefaciens carrying p35S::EMB1303-GFP or p35S::GFP plasmid was infiltrated into N. benthamiana leaves as described 34.
Quantitative RT-PCR
Total RNA was isolated from 3-week-old plants with TRI Reagents, followed by treatment with RNase-free DNase I (Promega, Madison, WI, USA) at 37 °C for 1 h to degrade genomic DNA. Treated RNA samples (1 μg each) were used as templates for first-strand cDNA synthesis (Promega). The resulting cDNAs were then subjected to PCR amplification using gene-specific primers. The PCR-amplified samples were separated on 1% (w/v) agarose gels. Three RT-PCR reactions were repeated independently using β-tubulin 8 (TUB8) as an internal control. Nucleotide sequences of gene-specific primers are available in Supplementary information, Table S3.
GUS assay
Histochemical detection of GUS activity was performed as previously described 33. Tissues for GUS staining were incubated in staining solution (50 mM sodium phosphate, pH 7.0, 10 mM EDTA, 2 mM 5-bromo-4-chloro-3-indoyl glucuronide, 1 mM potassium ferricyanide, and 1 mM potassium ferrocyanide) at 37 °C overnight. After incubation, stained tissues were cleared of chlorophyll in an ethanol series (20%, 40%, 60%, 80%, and 100%) and used for observation and photography.
Analysis of chlorophyll and carotenoids
Total chlorophylls and carotenoids were determined as described previously 37 with little modifications. Extracts were obtained from 21-day-old Arabidopsis seedlings grown on 1/2 MS medium. Fresh tissues were homogenized in 80% acetone. Spectrophotometric quantification was carried out in a Beckman DUR650 spectrophotometer. Pigment measurements were repeated in three independent experiments.
Protoplast preparations and chloroplast isolation
Protoplast preparations were performed as described 38. Briefly, protoplasts were prepared 3 days after infiltration by incubating leaf strips in buffer (500 mM mannitol, 10 mM CaCl2, 5 mM MES/KOH (pH 5.5), 3% cellulase, and 0.75% Macerozym). Intact chloroplasts were isolated as previously described 39 with modifications. Protoplasts were broken and centrifuged for 7 min at 1 000× g. The pellet was resuspended in buffer (0.45 M sorbitol, 50 mM Hepes-KOH pH 7.8, 2 mM EDTA, 0.1% BSA, 2.5 mM MgCl2, and protease inhibitor cocktail), and then layered on a discontinuous 40%/80% Percoll gradient. After centrifugation for 15 min at 7 000× g, intact chloroplasts were isolated at the interface of the two layers, followed by two washes in buffer (0.33 M sorbitol, 50 mM Hepes-KOH pH 7.8, 2.5 mM MgCl2, and protease inhibitor cocktail) with centrifugation for 5 min at 1 000× g.
Protein extraction and immunoblot analysis
Protein was isolated from tissues by pulverizing the tissue in ice-cold protein isolation buffer (60 mM Tris-HCl, pH 8.0, 50 mM NaCl, 10 mM EDTA, 30 mM β-mercaptoethanol, and 0.5 mM phenylmethanesulfonyl fluoride). Pulverized tissues were thoroughly mixed and cell debris was cleared by centrifugation at 12 000× g for 10 min at 4 °C. Protein concentrations were determined according to the method described 40 with bovine serum albumin as standard.
Extracts were fractionated by SDS-PAGE on 10% (w/v) gel (30:0.8 (w/w) acrylamide:bis-acrylamide) using a minigel system (Bio-Rad Laboratories, Richmond, CA, USA). After electrophoresis, the separated proteins were visualized by Coomassie Brilliant Blue G-250, or transferred electrophoretically onto a PVDF nitrocellulose membrane (Bio-Rad). After blotting, blots were incubated with antibodies at a 1:3 000 dilution. Immunodetection was carried out using the secondary antibody (horseradish peroxidase IgG (H+L)) and Western Blotting Luminol Reagent (Santa Cruz, CA, USA).
Fluorescence microscopy
The autofluorescence (red) of chloroplasts and fluorescence of GFP in the transformed N. benthamiana leaves or protoplasts were imaged using a confocal laser scanning microscope (LSM510, Carl Zeiss, Oberkochen, Germany) at 2-4 days after infiltration.
To detect chlorophyll autofluorescence, leaf samples were examined by using a fluorescence microscope (Olympus Z61, Japan) with a long-pass 590-nm filter set.
Light and electron microscopy
To examine the development of the embryos, ovules from different developmental stages were treated with a clearing solution of chloral hydrate, water, and glycerol (8:2:1, v/v) for 12 h. The developing embryos were then analyzed under a Nomarski microscope (Leica DM 2500, Japan).
Sections of leaf tissue were prepared for electron microscope analysis observation as previously described 10 with minor modifications. Briefly, Arabidopsis leaf tissue was fixed with glutaraldehyde followed by osmium tetroxide and dehydrated in an ethanol series before being infiltrated with Spurr's resin (Dow Chemical Co., USA). Polymerization was conducted at 70 °C for 8 h. Specimens were sliced to yield ultra-thin sections (LKB-8800, Sweden) and stained with uranyl acetate and alkaline lead citrate before being examined with a JEM-100S transmission electron microscope.
Microarray analysis
Affymetrix GeneChip ATH1 arrays representing ∼24 000 Arabidopsis genes were used for the analysis of whole genome gene expression profile in wild-type Col and emb1303-1 plants. Microarray experiments and data analysis were performed according to the manufacture's instruction (http://www.affymetrix.com/support/technical/manuals.affx). Briefly, total RNAs were extracted using the Plant RNA Prep Kit (Qiagen, Hilden, Germany) from 3-week-old seedlings of the mutant and the wild type grown on 1/2 MS medium containing 2% sucrose. In all, 5 μg of total RNA was used to synthesize Cy3- and Cy5-labeled cDNA, which was then hybridized with the ATH1 oligonucleotide chips as instructed by the manufacturer (Affymetrix). The raw microarray data were analyzed using Microarray Suite 5.0 software (Affymetrix).
( Supplementary Information is linked to the online version of the paper on the Cell Research website.)
References
Staehelin LA, Newcomb EH . Membrane structure and membranous organelles. In: Buchanan RB, Gruissem W, Jones RL, eds. Biochemistry and Molecular Biology of Plants. American Society of Plant Biology: Madison 2000:2–50.
Lopez-Juez E, Pyke KA . Plastids unleashed: their development and their integration in plant development. Int J Dev Biol 2005; 49:557–577.
Taylor WC . Regulatory interactions between nuclear and plastid genomes. Annu Rev Plant Physiol Plant Mol Biol 1989; 40:211–233.
Meurer J, Meierhoff K, Westhoff P . Isolation of high-chlorophyll-fluorescence mutants of Arabidopsis thaliana and their characterisation by spectroscopy, immunoblotting and northern hybridisation. Planta 1996; 198:385–396.
Plucken H, Muller B, Grohmann D, Westhoff P, Eichacker LA . The HCF136 protein is essential for assembly of the photosystem II reaction center in Arabidopsis thaliana. FEBS Lett 2002; 532:85–90.
Apuya NR, Yadegari R, Fischer RL, et al. The Arabidopsis embryo mutant schlepperless has a defect in the chaperonin-60α gene. Plant Physiol 2001; 126:717–730.
Motohashi R, Nagata N, Ito T, et al. An essential role of a TatC homologue of a Δ pH- dependent protein transporter in thylakoid membrane formation during chloroplast development in Arabidopsis thaliana. Proc Natl Acad Sci USA 2001; 98:10499–10504.
Sundberg E, Slagter JG, Fridborg I, et al. ALBINO3, an Arabidopsis nuclear gene essential for chloroplast differentiation, encodes a chloroplast protein that shows homology to proteins present in bacterial membranes and yeast mitochondria. Plant Cell 1997; 9:717–730.
Klostermann E, Droste Gen Helling I, Carde JP, Schunemann D . The thylakoid membrane protein ALB3 associates with the cpSecY-translocase in Arabidopsis thaliana. Biochem J 2002; 368:777–781.
Wang Y, Kollmann R . Vascular differentiation in the graft union of in-vitro grafts with different compatibility. Structural and functional aspects. J Plant Physiol 1996; 147:521–533.
Hsieh MH, Chang CY, Hsu SJ, Chen JJ . Chloroplast localization of methylerythritol 4-phosphate pathway enzymes and regulation of mitochondrial genes in ispD and ispE albino mutants in Arabidopsis. Plant Mol Biol 2008; 66:663–673.
Guevara-Garcia A, San Roman C, Arroyo A, et al. Characterization of the Arabidopsis clb6 mutant illustrates the importance of posttranscriptional regulation of the methyl-D-erythritol 4-phosphate pathway. Plant Cell 2005; 17:628–643.
Dong H, Deng Y, Mu J, et al. The Arabidopsis Spontaneous Cell Death1 gene, encoding a zeta-carotene desaturase essential for carotenoid biosynthesis, is involved in chloroplast development, photoprotection and retrograde signalling. Cell Res 2007; 17:458–470.
Qin G, Gu H, Ma L, et al. Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res 2007; 17:471–482.
Uwer U, Willmitzer L, Altmann T . Inactivation of a glycyl-tRNA synthetase leads to an arrest in plant embryo development. Plant Cell 1998; 10:1277–1294.
Apuya NR, Yadegari R, Fischer RL, Harada JJ, Goldberg RB . RASPBERRY3 gene encodes a novel protein important for embryo development. Plant Physiol 2002; 129:691–705.
Bellaoui M, Keddie JS, Gruissem W . DCL is a plant-specific protein required for plastid ribosomal RNA processing and embryo development. Plant Mol Biol 2003; 53:531–543.
Garcion C, Guilleminot J, Kroj T, et al. AKRP and EMB506 are two ankyrin repeat proteins essential for plastid differentiation and plant development in Arabidopsis. Plant J 2006; 48:895–906.
Patel R, Hsu SC, Bedard J, Inoue K, Jarvis P . The Omp85-related chloroplast outer envelope protein, OEP80, is essential for viability in Arabidopsis. Plant Physiol 2008; 148:235–245.
Kobayashi K, Suzuki M, Tang J, et al. LOVASTATIN INSENSITIVE 1, a novel pentatricopeptide repeat protein, is a potential regulatory factor of isoprenoid biosynthesis in Arabidopsis. Plant Cell Physiol 2007; 48:322–331.
Yu B, Wakao S, Fan J, Benning C . Loss of plastidic lysophosphatidic acid acyltransferase causes embryo-lethality in Arabidopsis. Plant Cell Physiol 2004; 45:503–510.
McElver J, Tzafrir I, Aux G, et al. Insertional mutagenesis of genes required for seed development in Arabidopsis thaliana. Genetics 2001; 159:1751–1763.
Ifuku K, Yamamoto Y, Ono TA, Ishihara S, Sato F . PsbP protein, but not PsbQ protein, is essential for the regulation and stabilization of photosystem II in higher plants. Plant Physiol 2005; 139:1175–1184.
Bang WY, Jeong IS, Kim DW, et al. Role of Arabidopsis CHL27 protein for photosynthesis, chloroplast development and gene expression profiling. Plant Cell Physiol 2008; 49:1350–1363.
Emanuelsson O, Nielsen H, von Heijne G . ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 1999; 8:978–984.
Oelmuller R . Photooxidative destruction of chloroplasts and its effect on nuclear gene expression and extraplastidic enzyme levels. Photochem Photobiol 1989; 49:229–239.
Koch KE . Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 1996; 47:509–540.
Borisjuk L, Nguyen TH, Neuberger T, et al. Gradients of lipid storage, photosynthesis and plastid differentiation in developing soybean seeds. New Phytol 2005; 167:761–776.
Gutierrez-Nava Mde L, Gillmor CS, Jimenez LF, Guevara-Garcia A, Leon P . CHLOROPLAST BIOGENESIS genes act cell and noncell autonomously in early chloroplast development. Plant Physiol 2004; 135:471–482.
Hajdukiewicz PT, Allison LA, Maliga P . The two RNA polymerases encoded by the nuclear and the plastid compartments transcribe distinct groups of genes in tobacco plastids. EMBO J 1997; 16:4041–4048.
Koussevitzky S, Nott A, Mockler TC, et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007; 316:715–719.
Nott A, Jung HS, Koussevitzky S, Chory J . Plastid-to-nucleus retrograde signaling. Annu Rev Plant Biol 2006; 57:739–759.
Yang S, Yang H, Grisafi P, et al. The BON/CPN gene family represses cell death and promotes cell growth in Arabidopsis. Plant J 2006; 45:166–179.
Walter M, Chaban C, Schutze K, et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 2004; 40:428–438.
Koncz C, Schell J . The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 1986; 204:383–396.
Clough SJ, Bent AF . Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 1998; 16:735–743.
Lichtenthaler HK, Wellburn AR . Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 1983; 11:591–592.
Yoo SD, Cho YH, Sheen J . Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2007; 2:1565–1572.
Barneche F, Winter V, Crevecoeur M, Rochaix JD . ATAB2 is a novel factor in the signalling pathway of light-controlled synthesis of photosystem proteins. EMBO J 2006; 25:5907–5918.
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248–254.
Acknowledgements
We thank Dr Lixin Zhang (Institute of Botany, Chinese Academy of Sciences) for providing antibodies of D1, D2, LHCII, and AtpB; and Arabidopsis Biological Research Center for T-DNA mutant seeds. We are grateful to Drs Jian Hua (Cornell University) and Hao Yu (National University of Singapore) for critically reviewing the manuscript. This work was supported by grants from National Natural Science Foundation of China (Nos. 30670181 and 30770202), the Ministry of Science and Technology of China (No. 2009CB119103), Program for New Century Excellent Talents in University (No. NCET-05-0124), and the Key Project of Chinese Ministry of Education (No. 106037).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information, Figure S1
Alignment of the deduced amino acid sequences of EMB1303 and its homologs from Arabidopsis (At1g30475), O. (PDF 16 kb)
Supplementary information, Figure S2
Morphology of emb1303-1 grown under different conditions. (PDF 121 kb)
Supplementary information, Figure S3
Chlorophyll autofluorescence of wild type and emb1303-1. (PDF 71 kb)
Supplementary information, Table S1
Functional classification of differentially expressed genes in emb1303-1. (PDF 82 kb)
Supplementary information, Table S2
Expression of genes involved in photosynthesis and chlorophyll biosynthesis in emb1303-1. (PDF 95 kb)
Supplementary information, Table S3
Gene-specific primers used in this study (PDF 45 kb)
Rights and permissions
About this article
Cite this article
Huang, X., Zhang, X. & Yang, S. A novel chloroplast-localized protein EMB1303 is required for chloroplast development in Arabidopsis. Cell Res 19, 1205–1216 (2009). https://doi.org/10.1038/cr.2009.84
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2009.84
Keywords
This article is cited by
-
OsAAI1 Increases Rice Yield and Drought Tolerance Dependent on ABA-Mediated Regulatory and ROS Scavenging Pathway
Rice (2023)
-
Comparative Physiological and Transcriptomic Analysis Provide New Insights of Crucial Pathways and Genes Regulating Kenaf Salt Tolerance
Journal of Plant Growth Regulation (2023)
-
The tomato WV gene encoding a thioredoxin protein is essential for chloroplast development at low temperature and high light intensity
BMC Plant Biology (2019)
-
High anthocyanin accumulation in an Arabidopsis mutant defective in chloroplast biogenesis
Plant Growth Regulation (2019)
-
The novel protein DELAYED PALE-GREENING1 is required for early chloroplast biogenesis in Arabidopsis thaliana
Scientific Reports (2016)