Abstract
Phospholipase D (PLD) plays a critical role in plant growth and development, as well as in hormone and stress responses. PLD encoding genes constitute a large gene family that are present in higher plants. There are 12 members of the PLD family in Arabidopsis thaliana and several of them have been functionally characterized; however, the members of the PLD family in Oryza sativa remain to be fully described. Through genome-wide analysis, 17 PLD members found in different chromosomes have been identified in rice. Protein domain structural analysis reveals a novel subfamily, besides the C2-PLDs and PXPH-PLDs, that is present in rice – the SP-PLD. SP-PLD harbors a signal peptide instead of the C2 or PXPH domains at the N-terminus. Expression pattern analysis indicates that most PLD-encoding genes are differentially expressed in various tissues, or are induced by hormones or stress conditions, suggesting the involvement of PLD in multiple developmental processes. Transgenic studies have shown that the suppressed expression of rice PLDβ1 results in reduced sensitivity to exogenous ABA during seed germination. Further analysis of the expression of ABA signaling-related genes has revealed that PLDβ1 stimulates ABA signaling by activating SAPK, thus repressing GAmyb expression and inhibiting seed germination.
Similar content being viewed by others
Introduction
Phospholipase D (PLD), which hydrolyzes phospholipids to produce phosphatidic acid (PA) and a free head group such as choline, has been detected in bacteria, fungi, plants and animals 1. Although PLD was found to be involved in lipid metabolism and membrane reconstruction in the 1940s 2, the first eukaryotic cDNA of PLD was cloned from the castor bean only in 1994 3. Since then, many PLD-encoding genes have been cloned from Arabidopsis thaliana 4, Oryza sativa 5, 6, Zea mays 5, Nicotiana tabacum 7 and Lycopersicon esculentum 8. Gene expression studies, protein domain structure analyses and biochemical characterization have greatly expanded our knowledge of the physiological functions and relevant regulations of PLD 9.
Biochemical studies have indicated that the phospholipid-hydrolyzing activities of PLD are either calcium-dependent (C2-PLD) or calcium-independent (PXPH-PLD) 10. Various phospholipid molecules, including phosphatidylinositol (PI), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG) and phosphatidylserine (PS), may be selectively hydrolyzed by different PLD members 11. Protein domain analysis has resulted in the identification of several conserved domains, including (1) the PLD-C1 and PLD-C2 domains, which are also known as the HKD (HxKxxxxD) domains and are responsible for the hydrolysis activity; (2) the C2 domain, the calcium/lipid-binding domain that is responsible for regulating Ca2+-dependent activity through binding to Ca2+; and (3) the PX (phox consensus sequence) and PH (pleckstrin homology) domains, which are located at the N-terminus of Ca2+-independent PLDs in place of the C2 domain of Ca2+-dependent PLDs 10.
Physiological studies have shown that PLDs are involved in multiple plant growth and developmental processes, such as seed germination, seedling growth, pollen tube germination and elongation, and leaf senescence 12, 13. Treatment with the PLD-specific inhibitor 1-butanol results in inhibited seed germination, altered emergence of the radicle and cotyledons, and abnormal root elongation and pattern formation of root hairs 14, 15. It was also shown that PLD and PA are critical for pollen germination and pollen tube elongation; reduced production of PA by PLD resulted in inhibited pollen tube germination and tip growth, and altered apical polarity of the pollen tube 16. In addition, the actin and microtubule structures were severely altered under 1-butanol treatment 14, 17, 18, 19.
Additionally, PLD and its product PA mediate the signaling of various plant hormones, including abscisic acid 20, 21, 22, 23, gibberellin 21, ethylene 20, cytokinin 24, jasmonic acid (JA) 25 and auxin 26. In Arabidopsis, treatment with 1-butanol represses cytokinin-induced ARR5-GUS expression 24 and suppresses the auxin response 26. Arabidopsis PLDα1 activity and PLDβ1 expression are wound-induced, and PLDα1 has been proven to be involved in ABA signal transduction and wound-induced JA accumulation 20, 25.
ABA is crucial in seed maturation, dormancy, germination and the cell response to stress 27. Recent genetic and biochemical approaches have enabled the identification of numerous components that are involved in ABA signaling, such as the ABA receptors FCA 28 and ABAR/CHLH 29, ABI1 30, 31, G protein 32 and PLD 21, 22, 33, 34. PLD and PA play an important role in ABA signaling. Suppressed PA or extra PA supply is able to either counteract or mimic the effects of ABA in barley aleuronic cells 21 and Vicia faba guard cells 33. Indeed, it has been shown that ABA stimulates PLD activity and increases the amount of PA produced 21, 23, 35, 36. In barley aleurone cells, PLD and PA mediate the inhibitory effects of ABA on GA-promoted α-amylase production 21. In Arabidopsis, the amount of PA is increased at an early stage of seed germination, and increased levels of PA through a deficiency in lipid phosphate phosphatase (LPP) lead to the reduced conversion of PA to diacylglycerol (DAG), which results in hypersensitive responses to ABA during seed germination 23. This is consistent with a previous report that the repressed expression of PLDα1 results in a decreased sensitivity to ABA and drought-induced stomatal closure 37.
Recent studies have shown that PLD and PA mediate plant responses to ABA by regulating the localization and activity of ABI1 22, a negative regulator of the ABA signaling pathway 30, 31. PA tethers ABI1 to the plasma membrane, which results in reduced translocation of ABI1 to the nucleus, and decreases ABI1 phosphatase activity 22. In addition, PLDα1 and PA are involved in ABA signaling through interaction with GPA1, a heterotrimeric GTP-binding protein (G protein) 34, 38.
Genome-wide analysis identified 12 PLD members in Arabidopsis 10, 39, and several members, including PLDα1, PLDδ, PLDζ1 and PLDζ2, were studied using biochemical or physiological approaches. PLDα1, the predominant PLD, is responsible for the common PLD activity in Arabidopsis, and is involved in ABA and ethylene signal transduction, freezing tolerance and wound-induced JA accumulation 13. PLDβ1 can bind to α-actin in vitro 40, and its expression is induced by wound stress 25. PLDδ is activated by oleic acid and is closely associated with the microtubule cytoskeleton and plasma membrane. It plays a positive role in the plant's response to different environmental stresses such as freezing and oxidative assault 13. PLDζ1 is involved in root hair pattern formation, and is a direct target of the homeobox transcription factor GLABRA2 (GL2) 15. Root elongation and digalactosyldiacylglycerol accumulation during phosphorus-limited growth conditions can be affected by PLDζ2 41, 42. Recently, our studies have shown that PLDζ2 expression is induced by IAA, and is required for the auxin response 26.
Environmental stimuli, such as drought and phosphate starvation, are critical factors affecting crop production, and the involvement of PLDs in these processes suggests their functional importance in crop growth. However, the presence and function of PLDs in rice, the model species for crops, remain to be fully studied. Here we present a detailed analysis of PLD genes in rice, and their phylogenetic relationship with their orthologs in Arabidopsis. In addition, our physiological studies showed that PLDβ1, a C2-PLD, is involved in seed germination through mediating ABA signal transduction.
Materials and Methods
Enzymes and chemicals
Enzymes used for DNA restriction and modification were obtained from Boehringer (Mannheim, Germany). IAA, GA, ABA and PA were obtained from Sigma-Aldrich (St Louis, MO, USA). The 'Trizol Kit' for RNA extraction was obtained from Invitrogen Company. DNA primers for polymerase chain reaction (PCR), Taq polymerase and a 'random labeling' kit were obtained from Genecore (Shanghai, China) and TaKaRa Biotechnology (Dalian, China). Nylon membranes and radiochemical [α-32P]dCTP were obtained from Amersham Pharmacia Biotech (USA) and Yahui Company (Beijing, China).
Bacteria and plant material
Escherichia coli DH5α cells were used for amplifying the cDNA library. The Agrobacterium tumefaciens EHA105 strain was used for rice transformation. Oryza sativa cv. Zhonghua 11 were germinated on 1/2 MS medium and grown in water in a phytotron with a 12-h light (26 °C) and 12-h dark (18 °C) period. For hormone and stress treatments, 2-week-old rice seedlings were treated with various hormones or stress conditions for 3 or 6 h (IAA, 100 μM; GA, 100 μM; ABA, 100 μM; NaCl, 250 mM, 29 oC).
Database search and sequence analysis
To identify members of the rice PLD family, multiple database searches were performed. First, we carried out a BLAST search of the TIGR rice annotation database (http://www.tigr.org/rice, Release 4), querying with the Arabidopsis PLD sequences. We also searched the annotation database using the gene name and conserved HKD domain (PF00614, PLD active site motif) as keywords. BLAST and keyword searches with the gene name were done for the Rice Annotation Project database (RAP, http://rapdb.lab.nig.ac.jp/cgi-bin/gbrowse/IRGSP, Build 4.0). In addition, the GRAMENE Rice Protein Database (http://www.gramene.org/) and the National Center for Biotechnology Information's GenBank (http://www.ncbi.nlm.nih.gov) were searched to identify PLD genes in rice. All the PLD genes identified have corresponding gene names in three other public rice databases, so the corresponding DNA and predicted PLD protein sequences from various database annotations were checked using ClustalW, with the default parameters as set by the European Bioinformatics Institute (EBI, http://www.ebi.ac.uk/clustalw/index.html). Finally, based on the protein sequence comparisons, the conserved domain composition and the phylogenetic relationship with Arabidopsis PLDs, all the rice PLD family genes were classified into different subgroups, and renamed with the uniform name.
The total number of ESTs was calculated from a GenBank database BLAST search. Hits from BLAST searches that had E-values above 0.1 were not considered for further analysis. The exon–intron structures of PLD genes were taken from the TIGR database and confirmed by comparing the cDNA with the corresponding genomic sequences. Domain and motif searches were carried out on the protein sequences in SMART (http://smart.embl-heidelberg.de/) and Pfam (http://www.sanger.ac.uk/software/pfam). Searching for targeting signals was performed using the TargetP program (http://www.cbs.dtu.dk/services/TargetP).
Expression pattern analysis via quantitative reverse transcription (RT)-PCR
Total RNA was isolated from seedlings, roots, stems, leaves and immature seeds or from harvested material treated with plant hormones and environmental stimuli. In all, 2 μg of total RNA was reverse transcribed according to the supplier's instructions (ReverTra plus, TOYOBO, Japan) and real-time RT-PCR was executed using the Rotor-Gene 3000 (Corbett Research, Sydney, Australia) with an SYBR green probe (SYBR Premix Ex Taq system, Takara). The amounts of amplified product were determined at the end of each cycle using the Rotor-Gene software (Ver. 6.0.16, Corbett Research). Rice actin1 (OSRAC1, X16280) was used as the internal positive control. The DNA primers used for qRT-PCR are listed as follows:
PLDα1-S (5′-TGG GTA ACC GTG AGG TGA AGC AG-3′) and PLDα1-A (5′-CCA TGG CGA TCT CAG AGT CCC TAG-3′);
PLDα2-S (5′-CGA CGC CGA CCC CAA GGA CTA CC-3′) and PLDα2-A (5′-TCG CCG ACC CGA CGA TGA TGT AC-3′);
PLDα3-S (5′-CTG ACC CGA GGG ATT ACC TTA CC-3′) and PLDα3-A (5′-CAT GGA CCT CTG GTT GAT GTT GG-3′);
PLDα4-S (5′-CTC AAG GCG AAG AGG ATG GAC G-3′) and PLDα4-A (5′-TGG CCG ATC CCA CGA TGA TGT AC-3′);
PLDα5-S (5′-AGC GAC GCC GAC CCG AGG GAT TA-3′) and PLDα5-A (5′-GAT GTT GGC CGA CCC GAC GAT GA-3′);
PLDβ1-S (5′-GGG TGC GTA TCA GCC ACA GTA T-3′) and PLDβ1-A (5′-CAT TAT CAA CAA ATC GTT CCC A-3′);
PLDβ2-S (5′-GAT CAA GTT CAG CCA ACA ATC CC-3′) and PLDβ2-A (5′-CAC AGT GAC ATC CTG TAC CCG TA-3′);
PLDδ1-S (5′-ATA CCG GCG TTT TAT GAT CTA TG-3′) and PLDδ1-A (5′-GAG GTC ATC AAC CAT CCC AAG A-3′);
PLDδ2-S (5′-TCG GAT CGG CCA ACA TCA ACC AG-3′) and PLDδ2-A (5′-TCC CTC ACC CGC CTC ACG CAC TC-3′);
PLDφ-S (5′-CCA CTG CAT GGG CAA GGT TGA GA-3′) and PLDφ-A (5′-ATG AGG TTG CTG GTG CCG ATG TT-3′);
Actin-1 (5′-GAA CTG GTA TGG TCA AGG CTG-3′) and Actin-2 (5′-ACA CGG AGC TCG TTG TAG AAG-3′).
Isolation of PLDβ1 cDNA
A rice EST clone (accession number C72286) that showed homology with PLD was found through an EST database search using Arabidopsis PLD (U84568) as bait. Based on the EST sequences, the specific primers PLD-1 (5′-GAT ACC CCG GCG TGC CC-3′) and PLD-2 (5′-TGG TCG GCG TCC CTG ATC-3′) were designed and used for isolating full-length cDNA from a library constructed of rice tiller material through PCR-based screening 43. Plaque purified phage clones were converted to pBluescript SK derivatives using the helper phage ExAssist according to the supplier's instructions (Stratagene, USA). The cloned pPLDβ1, which contained the longest cDNA insert, was used for further analysis. DNA sequencing was performed by Genecore Company (Shanghai, China).
RT-PCR and northern blot analysis of PLDβ1
RT-PCR analyses were carried out to examine PLDβ1 expression in different tissues. In all, 5 μg of total RNA, isolated from the roots, stem, leaves, spikes and immature seeds, was reverse transcribed and the resulting cDNAs were then used as templates for PCR amplification. The PLD-1 and PLD-2 primers were used. Rice actin1 was used as a positive internal control. A 2-week-old rice seedling was treated with 100 μM IAA and ABA at 0, 2, 4, 8 and 12 h and used for RNA extraction. A 900-bp fragment of PLDβ1 served as a [α-32P]dCTP-labeled hybridization probe.
Transgenic approach and rice regeneration
A 900-bp PLDβ1 fragment, digested from pPLDβ1 with SmaI and SalI, was subcloned to a p35S-1301 44 vector precut with the same enzymes. The resulting binary vector, p35S-1301-antiPLDβ1 harboring PLDβ in an antisense orientation, was transferred to the Agrobacterium strain EHA105 and used for rice transformation. Rice transformation and regenerated resistant lines were confirmed using the method described in Liu et al. 44. In total, 30 seeds from the confirmed T1 transgenic rice plants were germinated on hygromycin-supplemented selection medium for homozygous screening. The rice line in which all the seeds could germinate and grow normally was regarded as the homozygous line and was used for further analysis of the T2 generation.
Calculation of seed germination frequencies and observation of seedling growth
A total of 30 rice seeds from each homozygous transgenic line were germinated in medium supplemented with ABA (with concentrations at 10 or 20 μM) and in medium not supplemented with ABA. Seed germination frequencies were calculated after germination for 2, 3 and 4 days. All experiments were performed at least 3 times (n > 30). To examine the effects of PA on seed germination and seedling growth, 50 rice seeds were germinated and grown under 50 μM PA for 5 days. The primary root length and lateral root number were measured and statistically calculated. PA (1,2-diacyl-sn-glycero-3-phosphate sodium salt, P9511) was first dissolved in chloroform and dried under a stream of helium. It was then dispersed into deionized water by sonication and added to the medium.
Expression analysis of GAmyb, α-amylase, SAPK8 and SAPK10 by quantitative real-time RT-PCR
GAmyb, α-amylase, SAPK8 and SAPK10 expression was analyzed by quantitative real-time RT-PCR. The seeds were imbibed in water and incubated for 24 h, and then treated with PA (50 μM) or ABA (20 μM) for 24 h. Total RNA was extracted, 2 μg of total RNA was reverse transcribed and qRT-PCR was performed as described above. Rice actin1 was used as positive internal control. The primers used are listed as below:
GAmyb-1 (5′-CTG CGT TGC AGC CTA CTG AGT TA-3′) and GAmyb-2 (5′-TAC ATG GCG TAC CGA CAG AAG AA-3′);
α-amylase-S (5′-CGG TGA TGG CTA CGC AAT CTG GG-3′) and α-amylase-A (5′-ATT CGG ATC GGA TAC AGC TCG TT-3′);
SAPK8-S (5′-TAG TAT GAG CAG CCA GTA TGA GG-3′) and SAPK8-A (5′-TCT TGT TGG TCG ATG ACT TAC AT-3′);
SAPK10-S (5′-CTG TTC TTC ATT CGC AAC CAA AA-3′) and SAPK10-A (5′-ATC CTC AAA AGG ATA TGC ACC AA-3′).
Results
Identification of 17 PLD genes in Oryza sativa
To identify the rice PLD-coding genes, several approaches were used, including BLAST searches of databases (TIGR, Release 4; and RAP, Build 4.0) using Arabidopsis thaliana PLD genes as queries, keyword searches of the gene name “phospholipase” and ortholog searches using the conserved HKD domain (PF00614, PLD active site motif). The obtained results were combined and analyzed, resulting in the identification of 17 PLD members in rice. In particular, PLDκ (Os02g02790), which was inaccurately annotated as a C2 protein previously, was shown to indeed encode a C2-PLD.
There are different annotations and gene names in the various databases, and disparate names have been used by different researchers. These names are disordered and lead to confusion for further studies. To make it clear, we have classified the 17 PLD members into three subgroups and have applied a uniform name according to protein sequence comparisons, peptide structures (conserved domains) and phylogenetic relationships with their respective orthologs in Arabidopsis (Table 1). Transcriptions of rice PLD genes are supported by isolated cDNAs or Expressed Sequence Tags (ESTs), except in the case of PLDα7.
Structural organization and chromosomal distribution of rice PLDs
Based on the predicted sequences, obtained cDNAs, and corresponding ESTs, the exon–intron structure of each PLD coding gene was determined (Figure 1). It has been reported that the exon–intron junctions of PLD genes are highly conserved within the individual subgroups in Arabidopsis 10, 39; however, these features are not well conserved in rice. In Arabidopsis C2-PLDs have 4 or 10 exons and all PLDα genes have 4 exons. However, in rice PLDα genes have random exon numbers, i.e. 4 (PLDα6), 5 (PLDα7) or 3 (PLDα1, 2, 3, 4, 5, 8) (Figure 1). There are 10 exons in rice PLDβ and PLDδ, which are well conserved with those in Arabidopsis. PLDκ is a special C2-PLD gene; it has 6 exons and no orthologous gene in Arabidopsis.
Regarding the genes encoding PXPH-PLDs, which consist of various numbers of exons, PLDζ1 and ζ2 have 20 and 17 exons, respectively. This is similar to Arabidopsis, in which PLDζ1 and ζ2 have 21 and 16 exons, respectively. Rice PLDφ, the unique member of the third subfamily (see following section), has 7 exons.
In general, rice PLD genes are much larger than those in Arabidopsis and are distributed throughout 9 of the 12 chromosomes (they are not found on chromosomes 4, 11 and 12, Table 1). Interestingly, there is a tandem gene cluster located on chromosome 6 - PLDα3-PLDα4-PLDα5. These three genes share a very similar exon–intron structure, and are very similar at the protein level (>78% similarity, Table 2). This situation is analogous to the Arabidopsis PLDγ2–PLDγ1–PLDγ3 cluster (sharing over 92% similarity), suggesting that the PLD family genes in Arabidopsis and rice may share conserved evolution patterns.
Domain structural analysis identified three subfamilies of rice PLDs
Based on their structural organization, rice PLDs can be classified into the C2-PLD, PXPH-PLD and SP-PLD subfamilies (Table 2; Figure 2). All rice PLDs contain two conserved HKD domains and highly conserved 'HKD' sequences, except for PLDα7, which lacks 'HK' in the second HKD domain (data not shown).
Peptide domain structures of rice PLD members. Schematic representations of the conserved domain and motif structures of rice PLDs are shown. Domain and motif structures were determined through searching the PLD protein sequences in PFAM and SMART. C2, protein kinase C-conserved region 2; PX, phox domain; PH, pleckstrin homology domain; HKD, HxKxxxD, conserved catalytic region.
A conserved C2 domain is located at the N-terminus of C2-PLD. There is a long region (∼200 Aa) located in front of the C2 domain of rice PLDβ1 (Figure 2) that is not present in other PLDs. Similar long regions are also present in Arabidopsis PLDβ1 (NP_565963, 274 Aa) and upload cotton (Gossypium hirsutum) PLDβ1a (AAN05430, 274 Aa) and PLDβ1b (AAN05431, 352 Aa). However, the function of these long regions is still not known.
The PX and PH domains, located at the N-terminus of PXPH-PLDs, are highly conserved in both plants and animals. The most evident difference is that rice PLDζ1 has a shorter PH domain (25 Aa) than those of other PXPH-PLDs (rice PLDζ2, 93 Aa; Arabidopsis thaliana PLDζ1, 91 Aa; Arabidopsis thaliana PLDζ2, 114 Aa; Homo sapiens PLD1, 104 Aa; and Homo sapiens PLD2, 108 Aa) (Figure 2).
A signal peptide is detected at the N-terminus of PLDφ. As there is no other motif located in front of the first conserved HKD domain, PLDφ is therefore designated as an SP-PLD. A similar type of PLD is also found in Caenorhabditis elegans (CAE72017, NP_504824), Dictyostelium discoideum (XP_637114) and mammals (Homo sapiens PLD3, AAH00553; PLD4, AAH15003), but has not been identified in other higher plants, including Arabidopsis thaliana, wheat or maize. There is a “D” to “E” substitution in the second HKD domain of mammalian SP-PLDs, while such a situation is not present in rice PLDφ. This indicates that there is a long phylogenetic distance between rice and mammalian SP-PLDs. In addition, it is predicted that SP-PLDs are secreted, and that their subcellular location is very similar to the secreted phospholipase-sPLA2. However, there is still no report on the physiological functions of SP-PLDs. Other domains, including PIP2-binding domain, FIYIENQYF domain and HYG, have also been detected in rice PLD members (data not shown).
A comparative analysis further showed that PLD members from the same subfamily share high protein-level similarity (Table 2). Of the C2-PLDs, 8 PLDα members share over 55% similarity, 2 PLDβ members share 77% similarity and 3 PLDδ members share over 72% similarity. Two PLDζ members share 84% similarity, but have less similarity (< 55%) with other PLDs. PLDφ has low similarity with all other PLD members (<40%), except PLDα1 (67%) and PLDζ1 (80%).
Phylogenetic relationships of PLD genes in rice and Arabidopsis
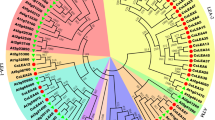
To study the evolutionary relationships between different PLD members, GrowTree was constructed using a GCG program to analyze the phylogenetic relationships of PLDs from different species. There is a close phylogenetic relationship between PLDα members, as well as PLDβ, PLDγ and PLDδ members, in rice and Arabidopsis. However, there is a long phylogenetic distance between Arabidopsis PLDɛ, rice PLDκ and other C2-PLDs (Figure 3A). Analysis of the PXPH-PLDs showed that rice PLDζ members have a close phylogenetic relationship with their Arabidopsis orthologs, but there is a long phylogenetic distance to their mammalian orthologs (Figure 3B). Additionally, there is a long distance from PLDφ to the mammalian PLDs (Figure 3C).
Phylogenetic relationships of PLD members in rice and Arabidopsis. GrowTrows were generated using the GCG program. The scale bars represent 100 amino acid substitutions per site. (A) A phylogenetic analysis of the relationship between C2-PLDs in rice and Arabidopsis. (B) A phylogenetic analysis of the relationship between PXPH-PLDs in rice, Arabidopsis and humans. (C) A phylogenetic analysis of the relationship between SP-PLDs in rice, humans and mice.
PLDs are expressed in various tissues and are involved in multiple cellular responses to hormones and environmental stimuli
Quantitative real-time RT-PCR analysis was performed to examine the expression patterns of PLD genes. As shown in Figure 4A, most PLD genes are expressed in a variety of tissues, including seedlings, roots, stems, leaves and immature seeds, although some of them have lower expression levels (expression levels of PLDα6-8, δ1-3 and κ are too low to be detected by qRT-PCR).
Expression of PLD genes in various tissues and their response to environmental factors measured by quantitative real-time RT-PCR analysis. (A) Transcript levels of PLD genes in various tissues of intact rice plants. (B–F) Relative expression of PLD genes during treatment with IAA (100 μM for 3 or 6 h, B), GA (100 μM for 3 or 6 h, C), ABA (100 μM for 3 or 6 h, D), salt (250 mM NaCl for 3 or 6 h, E) or drought (29 °C for 3 or 6 h, F).
Many rice PLD genes are induced by plant hormones (IAA, GA, ABA) or abiotic stress (salt and drought stress) (Figure 4B–4F). PLDα1 is induced by IAA, ABA and drought, and is suppressed by GA; PLDα2 is highly induced by salt and drought; PLDα3 is induced by most plant hormones and stress stimuli; while PLDα4 and PLDα5 are suppressed by most plant hormones and stress stimuli. PLDα1 is induced by most plant hormones and stress stimuli; and PLDβ1, PLDζ1 and PLDζ2 are induced by IAA, ABA and drought, and suppressed by salt stress. These differential expression patterns reveal the specific and distinct roles of different PLD genes in hormone effects and stress responses.
Isolation of PLDβ1, which encodes a C2-PLD and is induced by ABA
Of the 17 rice PLDs, only 5 have been cloned individually, and none of them has been functionally characterized yet. To elucidate the physiological function of PLDs in rice, cDNA encoding PLDβ1 was isolated. In short, a rice EST (C72286) sharing high similarity with PLDβ1 at amino acid positions 700–818 was identified by searching the dbEST database with the Arabidopsis PLDβ1 (U84568) coding sequence. Specific primers were then designed and used for cDNA library screening via PCR-based methods 43. The resulting cDNA clone (Accession No. AJ419630), with a length of 3180 bp and an open reading frame between nucleotides 478 (ATG) and 3015 (TGA), encodes a protein containing 845 AA. On comparing the fragment with the recently released full-length rice cDNA sequences, we found that this fragment is a partial cDNA of PLDβ1 (longer cDNA clones were isolated: AK073012 and AK121075 45).
Northern blot analyses indicated relatively low expression of PLDβ1 in various tissues, and further RT-PCR analysis showed that PLDβ1 is expressed in root, stem, leaf, spike and immature seeds (Figure 5A). In addition, northern blot analyses confirmed that PLDβ1 is rapidly induced by IAA and ABA (Figure 5B).
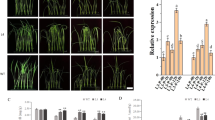
PLDβ1 deficiency results in reduced sensitivity to exogenous ABA. (A) Transcript levels of PLDβ1 in various tissues of intact rice plants, revealed through RT-PCR. R, root; S, stem; L, leaf; Sp, spike; and ImSe, immature seeds. (B) Northern blot analysis showed that both IAA (upper panel) and ABA (lower panel) stimulate PLDβ1 expression. Rice shoots (germinated for 10 days) were treated with 100 μM IAA for 0, 2, 4, 8 and 12 h, or 100 μM ABA for 0, 2, 4, 8 and 12 h. (C) Repressed expression of PLDβ1 in transgenic plants harboring pA-PLD. Total RNA was extracted from independent 14-day-old transgenic plants (lines 1, 2, 4, 6, 7, 11) and PLDβ1 transcripts were analyzed through RT-PCR. The Actin gene was used as an internal positive control. (D) Suppressed expression of PLDβ1 resulted in decreased ABA inhibition of seed germination and seedling growth. Seedlings were grown for 5 days under different concentrations of ABA (0, 10 or 20 μM; in the left panels the scale bar represents 1 cm). Germination ratios were measured and statistically calculated after treatment with ABA (0, 10 or 20 μM) for 72 h (right panel, upper) or at 10 μM (right panel, middle) or 20 μM (right panel, bottom) for different durations (48, 60, 72, 84 or 96 h). Error bars represent the SE (n > 30).
Deficiency of PLDβ1 results in repressed responses to exogenous ABA
A transgenic approach was used to study the physiological function of PLDβ1. A construct pA-PLD harboring PLDβ1 in an antisense orientation was transformed into rice using an immature embryo, and more than 10 resistant plants were obtained after initial screening. Harvested T1 seeds were germinated on medium supplemented with hygromycin to confirm the resistance. Single-copy integration of T-DNA was confirmed by the germination frequency (a 3:1 ratio) on selective medium, and further RT-PCR analysis confirmed the suppressed expression of PLDβ1 in transgenic plants (Figure 5C). The harvested T2 seeds were then used to identify homozygous lines for further study.
Under normal growth conditions, there is no evident difference between control and transgenic plants. As PLDβ1 is induced by ABA, and PLD has been shown to be involved in the ABA response in barely aleuronic cells 21 and Arabidopsis seed germination 23, 37, we focused on PLDβ1 effects in ABA responses. Phenotypic and statistical analysis of seed germination reveals a repressed sensitivity to exogenous ABA in PLDβ1-deficient plants (Figure 5D). Seed germination and seedling growth of wild-type plants are heavily inhibited by exogenous ABA, while the inhibitory effects are much reduced in PLDβ1-deficient plants.
PLDβ1 deficiency modulates the expression of SAPK, GAmyb and α-amylase
To further study the role of PLDβ1 and PA in ABA signaling transduction during seed germination, the effects of PA on seed germination and seedling growth were examined. In Arabidopsis, the amount of PA reaches a maximum at 12 h and decreases after 96 h during the early stages of seed germination. In addition, a deficiency of LPP results in a significant increase of PA and hypersensitive responses to ABA during seed germination 23. As shown in Figure 6A, long-term treatment with PA (50 μM for 5 days) inhibited seedling growth, especially primary root elongation and lateral root formation. This suggests a negative regulatory role for PA in seedling growth, which is consistent with the previous report that PA application will mimic the ABA effects 21.
PLDβ1 is involved in seed germination and seedling growth through the regulation of SAPK/PKABA and GAmyb expression. (A) PA represses rice seedling growth. Seedlings were grown on medium supplemented with 50 μM of PA and medium not supplemented with PA for 5 days (left). Primary root length (middle panel) and lateral root numbers (right panel) of 14-day-old seedlings were measured and statistically calculated (P < 0.01). Scale bar represents 1 cm. (B) PA (50 μM for 24 h) inhibits GAmyb and α-amylase expression, and stimulates SAPK8 and SAPK10 expression. (C) The relative expression of GAmyb, α-amylase, SAPK8 and SAPK10 in early seed germination without or with treatment of ABA in rice. PLDβ1 deficiency results in stimulated expression of GAmyb and α-amylase, while the expression of SAPK8 and SAPK10 is not altered (upper panel). Under treatment with ABA (20 μM), ABA-inhibited expression of GAmyb and α-amylase, and ABA-stimulated expression of SAPK8 and SAPK10 are much repressed (middle and lower panels). (D) A hypothetical model of the roles of PLDβ1 in ABA-regulated seed germination.
Studies using barley aleurone showed that PLD and its product PA are involved in the ABA-suppressed and GA-stimulated expression of GAmyb and α-amylase genes through regulating PKABA1, an ABA-activated SNF1 (sucrose nonfermenting protein kinases) type of protein kinase in barley 46, 47, 48, 49. In addition, Arabidopsis SnRK2 (SNF1-related protein kinase 2) is orthologous to PKABA1 50. SnRK2.6/OST1/SRK2E, the best characterized member of SnRK2 family, was activated by ABA and has essential roles in ABA signal transduction 51.
We thus tried to detect whether a similar regulatory mechanism operates during the process of rice seed germination. It has been shown that rice SAPK family genes are orthologous to PKABA1, and analysis of protein sequences and domain structures shows that rice SAPK8 and SAPK10 are most orthologous to PKABA1 and SnRK2.6/OST1/SRK2E, and, more importantly, that both of them are activated by ABA 50.
First, we studied the effects of PLDβ1 and PA on the expression of SAPK8 and SAPK10, GAmyb and α-amylase. As shown in Figure 6B, expression of SAPK8 and SAPK10 is stimulated by PA, while expression of GAmyb and α-amylase is clearly inhibited during treatment with PA, indicating that PA has similar effects as ABA on the expression of these genes. With PLDβ1 deficiency, the expression of GAmyb and α-amylase is clearly enhanced during early seed germination in rice (Figure 6C, upper panel), which is consistent with the observation that the expression of GAmyb and α-amylase is inhibited during treatment with PA. Further analysis shows that treatment with ABA (20 μM for 24 h) seriously suppresses the expression of GAmyb and α-amylase during seed germination in rice. However, the inhibitory effects are much reduced with PLDβ1 deficiency (Figure 6C, middle panel), which suggests the involvement of PLDβ1 in mediating the function of ABA. In addition, although expression of SAPK8 and SAPK10 was not significantly altered with PLDβ1 deficiency (Figure 6C, upper panel), the clearly enhanced expression of SAPK8 and SAPK10 by ABA was much reduced, similar to the untreated control (Figure 6C, lower panel), indicating a specific role for PLDβ1 in ABA-induced SAPK expression. This suggests that PLDβ1 is positively involved in the ABA-mediated inhibition of GAmyb and α-amylase expression, partially through SAPK regulation (Figure 6D).
Discussion
Although there are 17 PLDs in rice, only 6 of them have been isolated and studied for their expression patterns and subcellular locations 52, and only PLDβ1 has been physiologically characterized. The specific expression patterns of PLD genes may hint at their unique roles in plant growth and development.
PLD family in Oryza sativa
Through a reiterative database search of public databases, 17 PLD coding genes were identified in rice, and they were shown to share high sequence identity to their orthologs in Arabidopsis. The close phylogenetic relationship between rice PLDs and their orthologs in Arabidopsis suggests that the evolution of PLDs in different species is well conserved. Further exon–intron organizational studies indicate that the structures of rice PLD genes are more complex than their orthologs in Arabidopsis. Protein domain structure analysis reveals that all PLD members in rice, except PLDα7, contain two conserved HKD domains. PLDα7 has a mutation in the second HKD domain. Based on the domain structures, rice PLDs can be classified into three subfamilies, which include the C2-PLDs (14 members), the PXPH-PLDs (2 members) and the SP-PLD (1 member). This new type of PLD, SP-PLD, has a signal peptide rather than a C2 domain or PXPH domain at the N-terminus. It is predicted that it can be secreted out of the cell, and it is the first identified secreted PLD in higher plants. The specific localization of SP-PLD, just like sPLA2 in higher plants, suggests its specific physiological function in plant growth and developmental processes, such as plant defense. Interestingly, this type of PLD was identified in most animals, but in higher plants it was identified only in rice, revealing its unique role in PLD evolution.
Apart from PLDα7, rice PLD genes have been shown to be expressed in various tissues based on the support of isolated cDNAs or ESTs. There is no corresponding EST or cDNA support for PLDα7, indicating that PLDα7 maybe transcribed at a very low level or under specific conditions. Furthermore, PLDα7 might be an artificial gene, because there is no EST or cDNA support, and a 'D' to 'E' substitution in the second HKD domain might result in loss of protein function. Further quantitative real-time RT-PCR analyses confirm the expression of rice PLD genes in various tissues, although some of them are expressed at lower levels. Most PLD genes are induced by hormones (IAA, GA, ABA) or stress stimuli (salt and drought), indicating the possible involvement of PLDs in hormone effects and stress responses. In Arabidopsis, PLDs have been shown to be involved in multiple processes; for example, PLDα1 is involved in the signal transduction of ABA, GA, and ethylene, and in senescence, water loss and freezing tolerance; PLDδ1 is a positive regulator of stress response, and also acts as a bridge between the plasma membrane and microtubule 13, 16; PLDζ2 is involved in phosphorus-deficiency-induced root elongation and digalactosyldiacylglycerol accumulation 41, 42, and is required for auxin response 26. In rice, the distinct expression patterns of different PLD members in the presence of plant hormones and in stress responses reveal the multiple and specific roles of PLDs. This is consistent with previous studies. Previous studies have indicated that rice PLDs have an overlapping distribution but also have distinct expression patterns 52, which are induced by hydrogen peroxide 53 and are involved in elicitor-induced phytoalexin accumulation 52, thus indicating the specific and important roles of PLDs in plant growth and development.
PLDβ1 mediates ABA response and is involved in seed germination
Seed germination is a complex process controlled by many factors including light, temperature and plant hormones. GA is believed to promote seed germination, while ABA induces seed dormancy in maturing embryos and prevents seed germination 55, 56. Previous studies have shown that PLDs and PA are involved in ABA- and GA-regulated seed germination. PLD activity and the amount of PA are increased during seed germination and the seedling stage 23, 36, 57. In addition, PLD is one of the target proteins of ABA, and supplementation of PA into the endosperm of barley resulted in ABA-like inhibition of the GA response 21, indicating that PLD may involve or mediate ABA signal transduction during seed development.
Our studies show that rice PLDβ1 positively regulates the ABA response in seed germination. PLDβ1 deficiency results in decreased sensitivity to ABA during seed germination (Figure 5) and reduced tolerance to salt stress (Supplementary information, Figure S1), in a way similar to Arabidopsis PLDα1-deficient plants, which have decreased stomatal closure induced by ABA and reduced tolerance to drought stress 37. In addition, PA is able to mimic ABA function in activating the expression of SAPK8 and SAPK10, suppressing the expression of GAmyb and α-amylase, and inhibiting seedling growth (Figure 6), providing further evidence that PLD regulates the ABA response through its product PA, which is in agreement with previous investigations 21. Although PLDβ1 plays a positive role in the ABA response during seed germination, at present we cannot exclude the possibility that other PLD members also participate in the ABA response. Indeed, besides PLDβ1, several other rice PLD genes, including PLDα1, α3, β2 and δ2, can also be induced by ABA (Figure 4), which suggests that they might be involved in the ABA response as well.
In barley aleurone cells, PLD and PA are involved in ABA-suppressed and GA-stimulated GAmyb and α-amylase gene expression through the regulation of PKABA1 expression 46, 47, 48. Expression pattern analysis shows that SAPK8 and SAPK10, which are rice orthologs of PKABA1, are induced by ABA, and that this type of induction is repressed with PLDβ1 deficiency. This indicates that PLDβ1 is involved in the regulation of ABA-induced SAPK expression. However, the unaltered expression of SAPK8 and SAPK10 in PLDβ1-deficient rice in the absence of ABA suggests the presence of other mechanisms for controlling SAPK8 and SAPK10 expression. In addition, reduced inhibition of GAmyb and α-amylase expression by ABA in PLDβ1-deficient rice further confirms the negative roles of PLDβ1, as well as PA, in seed germination (Figure 6D).
Recent studies have shown that Arabidopsis SnRK2.10, an ortholog of rice SAPK and barely PKABA1 50, is the direct target protein of PA 58, revealing the possibility that SAPK may be the direct target protein of PA in rice. These results suggest that, during the process of seed germination, PLDβ1-derived PA may inhibit the expression of GAmyb and α-amylase directly or indirectly through activating SAPK functions, and thus may be negatively involved in seed germination (Figure 6D). In addition, it has been shown that ABA-induced activation of SnRK2.6/OST1/SRK2E, the closest Arabidopsis ortholog of SAPK8 and SAPK10, is inhibited by ABI1 through its binding to the conserved domain II at the N-terminus of SRK2E 57. Furthermore, as ABI1 activity is inhibited by PA 22, we may thus speculate that PLD and PA may activate SRK2E functions through inhibiting ABI1 activities. The presence of the conserved domain II at the SAPK8 and SAPK10 N-termini further supports this possibility; however, this needs to be further examined.
Expression of PLDβ1 is regulated not only by ABA but also by auxin, GA, salt and drought. The fact that PLDβ1-deficient plants showed increased sensitivity to salt (Supplementary information, Figure S1) and treatment with GA (data not shown) suggests that PLDβ1 might be involved in the effects of multiple plant hormones and in stress tolerance.
Accession numbers
Sequence data from this article can be found on the GenBank website. Accession numbers for rice PLDs are listed in Table 1. For Arabidopsis PLDs, please refer to Qin and Wang (2002) 10. Other sequences are listed as follows: hPLD1 (AAH68976), hPLD2 (AAB96655), hPLD3 (AAH00553), hPLD4 (AAH15003), MmPLD3 (AAH76586), MmPLD4 (AAH58565), GAmyb (OsGAMYB, X98355), α-amylase (OsALAM, X16509), SAPK8 (AB125309) and SAPK10 (AB125311).
References
Morris AJ, Engebrecht J, Frohman MA . Structure and regulation of phospholipase D. Trends Pharmacol Sci 1996; 17:182–185.
Hanahan DJ, Chaikoff IL . A new phospholipid splitting enzyme specific for the ester linkage between the nitrogenous base and the phosphoric acid grouping. J Biol Chem 1947; 169:699–705.
Wang X, Xu L, Zheng L . Cloning and expression of phosphatidylcholine-hydrolyzing phospholipase D from Ricinus communis L. J Biol Chem 1994; 269:20312–20317.
Qin W, Pappan K, Wang X . Molecular heterogeneity of phospholipase D (PLD). Cloning of PLDgamma and regulation of plant PLDgamma, -beta, and -alpha by polyphosphoinositides and calcium. J Biol Chem 1997; 272:28267–28273.
Ueki J, Morioka S, Komari T, Kumashiro T . Purification and characterization of phospholipase D (PLD) from (Oryza sativa L.) and cloning of cDNA for PLD from rice and maize (Zea mays L.). Plant Cell Physiol 1995; 36:903–914.
Morioka S, Ueki J, Komari T . Characterization of two distinctive genomic clones (accession nos. AB001919 and AB001920) for phospholipase D from rice (PGR 97–076). Plant Physiol 1997; 114:396.
Lein W, Saalbach G . Cloning and direct G-protein regulation of phospholipase D from tobacco. Biochim Biophys Acta 2001; 1530:172–183.
Whitaker BD, Smith DL, Green KC . Cloning, characterization and functional expression of a phospholipase Dalpha cDNA from tomato fruit. Physiol Plant 2001; 112:87–94.
Wang X . Multiple forms of phospholipase D in plants: the gene family, catalytic and regulatory properties, and cellular functions. Prog Lipid Res 2000; 39:109–149.
Qin C, Wang X . The Arabidopsis phospholipase D family characterization of a calcium-independent and phosphatidylcholine-selective PLDζ1 with distinct regulatory domains. Plant Physiol 2002; 128:1057–1068.
Munnik T, Irvine RF, Musgrave A . Phospholipid signalling in plants. Biochim Biophys Acta 1998; 1389:222–272.
Wang X . Phospholipase D in hormonal and stress signaling. Curr Opin Plant Biol 2002; 5:408–414.
Wang X . Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol 2005; 139:566–573.
Gardiner J, Collings DA, Harper JD, Marc J . The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organization in Arabidopsis. Plant Cell Physiol 2003; 44:687–696.
Ohashi Y, Oka A, Rodrigues-Pousada R, et al. Modulation of phospholipid signaling by GLABRA2 in root–hair pattern formation. Science 2003; 300:1427–1430.
Monteiro D, Liu Q, Lisboa S, Scherer GE, Quader H, Malho R . Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c and membrane secretion. J Exp Bot 2005; 56:1665–1674.
Dhonukshe P, Laxalt AM, Goedhart J, Gadella TWJ, Munnik T . Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell 2003; 15:2666–2679.
Motes CM, Pechter P, Yoo CM, Wang YS, Chapman KD, Blancaflor EB . Differential effects of two phospholipase D inhibitors, 1-butanol and N-acylethanolamine, on in vivo cytoskeletal organization and Arabidopsis seedling growth. Protoplasma 2005; 226:109–123.
Hirase A, Hamada T, Itoh TJ, Shimmen T, Sonobe S . n-Butanol induces depolymerization of microtubules in vivo and in vitro. Plant Cell Physiol 2006; 47:1004–1009.
Fan L, Zheng S, Wang X . Antisense suspension of PLD alpha retards abscisic acid and ethylene-promoted senescence of postharvest Arabidopsis. Plant Cell 1997; 9:2183–2196.
Ritchie S, Gilroy S . Abscisic acid signal transduction in the barley aleurone is mediated by phospholipase D activity. Proc Natl Acad Sci USA 1998; 95:2697–2702.
Zhang W, Qin C, Zhao J, Wang X . Phospholipase Dá1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA 2004; 101:9508–9513.
Katagiri T, Ishiyama K, Kato T, Tabata S, Kobayashi M, Shinozaki K . An important role of phosphatidic acid in ABA signaling during germination in Arabidopsis thaliana. Plant J 2005; 43:107–117.
Romanov GA, Kieber JJ, Schmulling T . A rapid cytokinin response assay in Arabidopsis indicates a role for phospholipase D in cytokinin signalling. FEBS Lett 2002; 515:39–43.
Wang C, Zien CA, Afitlhile M, Welti R, Hildebrand DF, Wang X . Involvement of phospholipase D in wound-induced accumulation of jasmonic acid in Arabidopsis. Plant Cell 2000; 12:2237–2246.
Li G, Xue HW . Arabidopsis PLDζ2 regulates vesicle trafficking and is required for auxin response. Plant Cell 2007; 19:281–295.
Finkelstein RR, Gampala SS, Rock CD . Abscisic acid signaling in seeds and seedlings. Plant Cell 2002; 14(Suppl):S15–S45.
Razem FA, El-Kereamy A, Abrams SR, Hill RD . The RNA-binding protein FCA is an abscisic acid receptor. Nature 2006; 439:290–294.
Shen YY, Wang XF, Wu FQ, et al. The Mg-chelatase H subunit is an abscisic acid receptor. Nature 2006; 443:823–826.
Sheen J . Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc Natl Acad Sci USA 1998; 95:975–980.
Gosti F, Beaudoin N, Serizet C, Webb A, Vartanian N, Giraudat J . ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 1999; 11:1897–1909.
Wang XQ, Ullah H, Jones AM, Assmann SM . G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 2001; 292:2070–2072.
Jacob T, Ritchie S, Assmann SM, Gilroy S . Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci USA 1999; 96:12192–12197.
Mishra G, Zhang W, Deng F, Zhao J, Wang X . A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 2006; 312:264–266.
Zalejski C, Zhang Z, Quettier AL, et al. Diacylglycerol pyrophosphate is a second messenger of abscisic acid signaling in Arabidopsis thaliana suspension cells. Plant J 2005; 42:145–152.
Ritchie S, Gilroy S . Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein-mediated and localized to the plasma membrane. Plant Physiol 2000; 124:693–702.
Sang Y, Zheng S, Li W, Huang B, Wang X . Regulation of plant water loss by manipulating the expression of phospholipase Dalpha. Plant J 2001; 28:135–144.
Zhao J, Wang X . Arabidopsis phospholipase Dα1 interacts with the heterotrimeric G-protein α-subunit through a motif analogous to the DRY motif in G-protein-coupled receptors. J Biol Chem 2004; 279:1794–1800.
Elias M, Potocky M, Cvrckova F, Zarsky V . Molecular diversity of phospholipase D in angiosperms. BMC Genomics 2002; 3:2.
Kusner DJ, Barton JA, Qin C, Wang X, Iyer SS . Evolutionary conservation of physical and functional interactions between phospholipase D and actin. Arch Biochem Biophys 2003; 412:231–241.
Li M, Qin C, Welti R, Wang X . Double knockouts of phospholipases Dzeta1 and Dzeta2 in Arabidopsis affect root elongation during phosphate-limited growth but do not affect root hair patterning. Plant Physiol 2006; 140:761–770.
Li M, Welti R, Wang X . Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of phospholipases D zeta1 and D zeta2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol 2006; 142:750–761.
Alfandari D, Darribere TA . Simple PCR method for screening cDNA libraries. PCR Meth Appl 1994; 4:46–49.
Liu W, Xu ZH, Luo D, Xue HW . Roles of OsCKI1, a rice casein kinase I, in root development and plant hormone sensitivity. Plant J 2003; 36:189–202.
Kikuchi S, Satoh K, Nagata T, et al. Collection, mapping, and annotation of over 28,000 cDNA clones from japonica rice. Science 2003; 301:376–379.
Gomez-Cadenas A, Verhey SD, Holappa LD, Shen Q, Ho TH, Walker-Simmons MK . An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proc Natl Acad Sci USA 1999; 96:1767–1772.
Gomez-Cadenas A, Zentella R, Walker-Simmons MK, Ho TH . Gibberellin/abscisic acid antagonism in barley aleurone cells: site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. Plant Cell 2001; 13:667–679.
Shen Q, Gomez-Cadenas A, Zhang P, Walker-Simmons MK, Sheen J, Ho TH . Dissection of abscisic acid signal transduction pathways in barley aleurone layers. Plant Mol Biol 2001; 47:437–448.
Yamauchi D, Zentella R, Ho TH . Molecular analysis of the barley (Hordeum vulgare L.) gene encoding the protein kinase PKABA1 capable of suppressing gibberellin action in aleurone layers. Planta 2002; 215:319–326.
Kobayashi Y, Yamamoto S, Minami H, Kagava Y, Hattori T . Differential activation of the rice sucrose nonfermenting1 related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 2004; 16:1163–1177.
Yoshida R, Hobo T, Ichimura K, et al. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 2002; 43:1473–1483.
McGee JD, Roe JL, Sweat TA, Wang X, Guikema JA, Leach JE . Rice phospholipase D isoforms show differential cellular location and gene induction. Plant Cell Physiol 2003; 44:1013–1026.
Yamaguchi T, Tanabe S, Minami E, Shibuya N . Activation of phospholipase D induced by hydrogen peroxide in suspension-cultured rice cells. Plant Cell Physiol 2004; 45:1261–1270.
Yamaguchi T, Minami E, Ueki J, Shibuya N . Elicitor-induced activation of phospholipases plays an important role for the induction of defense responses in suspension-cultured rice cells. Plant Cell Physiol 2005; 46:579–587.
Bewley JD . Seed germination and dormancy. Plant Cell 1997; 9:1055–1066.
Finch-Savage WE, Leubner-Metzger G . Seed dormancy and the control of germination. New Phytol 2006; 171:501–523.
Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K . The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 2006; 281:5310–5318.
Testerink C, Dekker HL, Lim ZY, et al. Isolation and identification of phosphatidic acid targets from plants. Plant J 2004; 39:527–536.
Acknowledgements
This work was supported by the State Key Project of Basic Research (2005CB120803) and the National Natural Science Foundation of China (30425029, 30421001). We thank Ms Shu-Ping Xu (Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for help on rice transformation.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Li, G., Lin, F. & Xue, HW. Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLDβ1 in seed germination. Cell Res 17, 881–894 (2007). https://doi.org/10.1038/cr.2007.77
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2007.77
Keywords
This article is cited by
-
Genome-wide in silico identification of phospholipase D (PLD) gene family from Corchorus capsularis and Corchorus olitorius: reveals their responses to plant stress
Journal of Genetic Engineering and Biotechnology (2022)
-
Genome-wide investigation of the PLD gene family in alfalfa (Medicago sativa L.): identification, analysis and expression
BMC Genomics (2022)
-
Structural insights into PA3488-mediated inactivation of Pseudomonas aeruginosa PldA
Nature Communications (2022)
-
Genome-wide identification and expression analysis of phospholipase D gene in leaves of sorghum in response to abiotic stresses
Physiology and Molecular Biology of Plants (2022)
-
Genome-wide association study (GWAS) of germination and post-germination related seedling traits in rice
Euphytica (2022)