Abstract
Parthenogenetic embryonic stem (pES) cells provide a valuable in vitro model system for studying the molecular mechanisms that underlie genomic imprinting. However, the pluripotency of pES cells and the expression profiles of paternally expressed imprinted genes have not been fully explored. In this study, three mouse pES cell lines were established and the differentiation potential of these cells in extended culture was evaluated. The undifferentiated cells had a normal karyotype and homozygous genome, and expressed ES-cell-specific molecular markers. The cells remained undifferentiated after more than 50 passages and exhibited pluripotent differentiation capacity. All three lines of the established ES cells produced teratomas; two lines of ES cells produced chimeras and germline transmission. Furthermore, activation of the paternally expressed imprinted genes Snrpn, U2af1-rs1, Peg3, Impact, Zfp127, Dlk1 and Mest in these cells was detected. Some paternally expressed imprinted genes were found to be expressed in the blastocyst stage of parthenogenetically activated embryos in vitro and their expression level increased with extended pES cell culture. Furthermore, our data show that the activation of these paternally expressed imprinted genes in pES cells was associated with a change in the methylation of the related differentially methylated regions. These findings provide direct evidence for the pluripotency of pES cells and demonstrate the association between the DNA methylation pattern and the activation of paternally expressed imprinted genes in pES cells. Thus, the established ES cell lines provide a valuable model for studying epigenetic regulation in mammalian development.
Similar content being viewed by others
Introduction
Embryonic stem (ES) cells are derived from early pre-implantation embryos 1, 2. These cells have the capacity to give rise to differentiated progeny that represent all three embryonic germ layers and can proliferate indefinitely in vitro. Therefore, these cells provide a unique tool for studying both biological and medical issues. The establishment of human ES cell lines and the demonstration of their differentiation potential in vitro have increased our interest in the potential use of these cells as a source of differentiated cells for repairing human degenerative or damaged tissues 3. However, there are many obstacles that hinder the development of these cell-based transplantation therapies. One such obstacle is immune rejection 4. Immune rejection results from the expression of the major histocompatibility complex (MHC) genes. Although it is possible to reduce immune-mediated rejection by carefully matching MHC genes between donor and recipient, finding a perfect match between them in the general population is very difficult, if not impossible, considering the high level of polymorphism for each MHC gene, which is made even more unlikely by heterozygosity. Creating genetically matched ES cells could be a strategy to overcome immunorejection. Unfertilized mammalian MII oocytes can be artificially activated to develop into blastocysts, from which the inner cell mass (ICM) can be isolated and parthenogenetic embryonic stem (pES) cells can be derived. In contrast to ES cells that are derived from fertilized embryos (with heterozygous MHC genes), pES cells are either uniformly homozygous or have minimal crossover-associated heterozygosity. With such a procedure, there is a greater likelihood of obtaining a match between the differentiated cells and the recipient. Recently, Kim et al. 5 demonstrated that selected pES cells can serve as a source of histocompatible tissues for transplantation. Although both mouse and primate pES cells have been demonstrated to undergo extensive differentiation in vitro 6, 7, and to contribute to a variety of adult tissues in chimeric mice 8, there are some discrepancies regarding the proliferation capacities of pES cells 7, 9, 10, 11. Therefore, it is necessary to fully explore the developmental potential of pES cells both in vitro and in vivo.
Both parental genomes are required for the successful development of mammals 12. This is due to genomic imprinting, an epigenetic phenomenon that results in some genes being expressed according to their parental origin. It is established in the germ line and is stably inherited throughout somatic cell division 13, 14. Approximately 50 known imprinted genes have been identified in humans 15. These genes have been shown to play important roles in the control of pre- and postnatal growth, and in the development of particular lineages and certain diseases 16, 17. Theoretically, paternally expressed imprinting genes should not be transcribed in pES cells. However, there have been reports about the disrupted expression of some of these genes 18. In addition, it has been reported that expression of some imprinted genes in mouse ES cells becomes unstable during in vitro culture 19. Therefore, it is very important to examine the integrity of imprinting in ES cells, especially by investigating the underlying mechanisms that regulate expression of imprinted genes.
In the current study, three mouse pES cell lines were established from chemically activated MII oocytes in order to investigate the developmental capacity of pES cells and to use these cells to study imprinted genes. The established cell lines remained undifferentiated after more than 50 passages. Meanwhile, the cells retain pluripotent differentiation potential both in vitro and in vivo. They are germline-competent. To our knowledge, these are the most extensively cultured and systemically characterized mouse pES cell lines. The expression patterns of several paternally expressed imprinting genes were also studied. More importantly, the study establishes the correlation between the activation of imprinting genes and the alteration of genome methylation in pES cells and reports for the first time that the activation of some of paternally expressed genes can be detected at the blastocyst state of parthenogenetic embryos. The established cell lines can be used as models for investigating the mechanisms of genome imprinting regulation and they are free for academic researchers.
Materials and Methods
Oocyte collection and parthenogenetic activation
C57BL/6, DBA/2J and CBA mice were purchased from Shanghai laboratory animal center and care of the mice was in accordance with the Guidelines of Shanghai Second Medical University for the Use of Animals in Research. Four-week-old female mice, C57BL/6 × DBA/2J F1 and C57BL/6 × CBA F1, were super-ovulated by intraperitoneal injection of 5 units of the pregnant mares' serum (PMS) gonadotropin, followed 48 h later by intraperitoneal injection of 5 units of human chorionic gonadotropin (HCG). Mature oocytes were collected from oviducts 17 h after HCG injection. They were freed from the cumulus cells by treatment with 0.1% hyaluronidase for 1 min (Sigma). Oocytes were washed with MHTF (Irvine Scientific), followed by exposure for 2 min to 5 μM ionomycin (Calbiochem) at room temperature and incubation for 4 h with 2 mM 6-dimethylaminopurine (6-DMAP, Sigma) at 5% CO2 and 37 °C to inhibit formation of the second polar body 20, 21.
Derivation of mouse pES cell lines
The treated oocytes were washed extensively in MHTF and cultured in P1 medium (Irvine Scientific; supplemented with a 10% serum substitute). Embryos were transferred to a BM medium (Irvine Scientific; supplemented with a 10% serum substitute) at day 3 and cultured to expanded blastocysts. Zona pelucidae were removed by pronase (5 mg/ml, Sigma) digestion for 2 min. Blastocysts were transferred to mitomycin C-treated ICR mouse feeder cells in gelatinized tissue culture wells (one embryo per well) and cultured for 4 days in an ES-cell medium as described 22. ICM outgrowths were mechanically dissociated into clumps with a finely drawn glass capillary and re-plated on fresh feeder cells. The propagating colonies were further passaged following exposure to 0.05% trypsin/EDTA.
Mouse ES cells derived from normally fertilized embryos
The ES cells derived from normally fertilized embryos that were used in the current study include D3 (purchased from ATCC) and WT (derived from C57BL/6 × DBA/2J blastocysts in our laboratory) cell lines.
Characterization of mouse pES cell lines
To confirm homozygosity in established lines, polymerase chain reaction (PCR)-based haplotype analyses were carried out using the microsatellite markers D16mit9, D1mit295, D3mit149, D4mit152, D6mit159, D5mit345 and D7mit267 (primer sequences can be obtained at http://www.informatics.jax.org/; the M13 19 bp sequence 5′-CAC GAC GTT GTA AAA CGA C-3′ was added to a 5′ primer as a fluorescent tag). Cytogenetic analysis of all ES cell lines was carried out using the standard protocol. At least 100 metaphases from each of the cell lines were studied in order to establish their chromosome number. Alkaline phosphatase (AKP) activity was detected with the vector blue substrate kit (Vector Laboratories). Telomerase activity was measured with the TRAPeze Telomerase Detection kit (Intergen) as recommended by the manufacturer. Oct-4 was detected by immunocytochemistry with rabbit affinity-purified polyclonal antibody generated in our laboratory 22 and localized with biotinylated secondary antibodies and then an avidin/biotinylated horseradish peroxidase complex (Vectastain ABC system, Vector Laboratories). Anti-stage-specific embryonic antigen-1 (SSEA-1, MC480, Chemicon) and fluorescein isothiocyanate (FITC)-conjugated secondary antibodies were used to detect SSEA-1.
In vitro differentiation
To generate embryoid bodies (EBs), ES cells were trypsinized, dispersed into a single-cell suspension, and then transferred to standard gelatinized 10 cm tissue culture dishes. They were incubated at 37 °C for 30 min to allow for removal of feeder cells by differential adherence. Then, the fibroblast-free suspension was diluted with ES cell medium without LIF to a concentration of 2.5 × 104 cells/ml. Hanging drops containing approximately 500 cells in 20 μl were maintained for 2-3 days on the lids of dishes filled with PBS. The resulting cell aggregates were denoted as EBs 23. For in vitro spontaneous differentiation, the formed EBs were transferred into four-well plates and cultivated in ES medium without LIF for 10 to 20 days and the spontaneously differentiated EBs were processed for immunocytochemical analysis. For in vitro induced neural differentiation, the EBs from hanging drops were transferred into dishes and cultivated for 3 days in medium without LIF, followed by being induced with 10−6 to 10−5 M retinoic acid (RA, Sigma) for an additional 3 days. The medium was then exchanged for a RA-free fresh medium. After 3 to 5 days, the differentiated EBs were fixed in 4% paraformaldehyde at room temperature for 15 min, followed by permeabilization for 10 min in 0.2% Triton X-100 in PBS. Then, the cells were blocked with 2.5% BSA in PBS at room temperature for 30 min, followed by incubation at 4 °C overnight with GATA4 antibody (1:100; Santa Cruz), Desmin antibody (1:50; DAKO), Map2 antibody (1:500; Santa Cruz), Nestin antibody (1:100; Chemicon), GFAP antibody (1:1 000; DAKO) and TuJ-1(1:200; Sigma) in PBS. After washing, cells were incubated with the appropriate secondary antibodies conjugated to Cy3 (1:100; Sigma) or FITC (1:100; Jackson) at room temperature for 1 h. The cell nucleus was labeled by DAPI (1:2000; Sigma). Cells were then washed in PBS and mounted for examination under a fluorescence microscope.
In vivo differentiation
To form teratomas approximately 5 × 106 cells were injected into the rear leg muscles of 5-week-old male SCID-Beige mice (two mice per cell line). Four to six weeks after cell injection, the resulting teratomas were examined histologically. Sections were stained with hematoxylin/eosin.
Chimera generation and germline transmission
Sp3 and Sp6 ES cells were trypsinized and feeder cells were removed. For microinjection, 10 blastocysts of ICR mice were placed in a drop of DMEM-HEPES with 15% FCS under mineral oil. The injection pipette containing 15 ES cells was pressed against the zona opposite the inner-cell mass. A brief pulse of the Piezo (Primetech, Ibaraki, Japan) was applied, and the injection needle was simultaneously pushed through the zona and trophectoderm layer into the blastocyst cavity. The ES cells then were expelled from the injection pipette and pushed against the inner-cell mass of the blastocyst. After the entire group was injected, the blastocysts were returned to the DMEM with 15% FCS and placed at 37 °C until their transfer to recipient females. Seven injected blastocysts were transferred to each uterine horn of the 2.5-d.p.c. pseudopregnant C57BL/6 × CBA F1 female mice. To produce germline transmitted mice, the chimeras were back crossed with wild-type (WT) ICR mice.
RT-PCR
At each passage, the trypsin-digested cells were incubated in the dish for 30 min to allow feeder cells to attach to the plates; then ES cells were then harvested carefully. D3 and Sp3 ES cells that were passaged in this way for 6, 7 and 8 times were used for analyzing imprinted genes. The first strand of cDNA was synthesized using 2 μg of total RNA from pES cells and D3 mouse ES cells by M-MLV reverse transcriptase (Promega). 1/25 of the reverse transaction product was used in RT-PCR. Several primer sets were used. These include Snrpn forward: TTC TTA GCT GAG ACA CCA AGA and Snrpn reverse: GAA GGT GCC AAT GAA GAT TCT C; U2af1-rs1 forward: GAT CAG ACA TAC TGC GGA TA and U2af1-rs1 reverse: GT GGT ACG GCC AGC CTA TG; H19 forward: TGC CTG ACC CGG GAG ACC ACC AC and H19 reverse: GCT ATC TCC GGG ACT CCA AAC CAG; and Hprt forward: TCA GTC AAC GGG GGA CAT AAA and Hprt reverse: TCA GTC AAC GGG GGA CAT AAA. The same procedure was carried out with Sp34 (p8) and Sp6 (p8) cDNA to detect expression of the imprinted genes in these lines.
Genomic DNA PCR
Genomic DNA was extracted with the UNIQ-10 column (Sengon) according to the manufacturer's instruction. The forward PCR primer for the Zfy gene is AAG ATA AGC TTA CAT AAT CAC ATG GA and the reverse primer is CCT ATG AAA TCC TTT GCT GCA CAT GT. The forward primer Gapdh is AAG CCA AAC TAG CAG CTA GG and the reverse primer for Gapdh is GGG CTA GTC TAT CAT TGC AG. The Zfy PCR was cycled for 35 times to ascertain the presence or absence of Y chromosomal DNA.
Quantitative RT-PCR
The primers were designed using the Primer Express 2 software (ABI) and are shown in the Supplementary information, Table 2. Real-time PCR was performed with water blank negative controls and each sample was analyzed in triplicate with β-actin as the inner control. The final PCR reaction volume of 10 μl contained 5 μl of SYBR® Green PCR Master Mix (ABI), 2 μl of 1:4 diluted cDNA template and 3 μl of primer mixture (containing 250 nM of each primer). Thermal cycling was carried out with a 10-min denaturation step at 95 °C, followed by 40 two-step cycles of 15 s at 95 °C and 60 s at 60 °C. Amplification data were collected by the ABI PRISM 7900 and analyzed by the Sequence Detection System 2.0 software (ABI). The expression level of each imprinted gene was normalized by the inner control, and the expression ratio of parthenogenetic Sp3 to D3 or the WT was presented.
Nest-PCR
The primers used for transcriptional analysis in mouse pre-implantation embryos were all designed across introns to rule out the expansion of genomic DNA. The sequences of the primers are listed in Supplementary information, Table 1. Single blastocysts (C57BL/6 × DBA/2J) that were collected from either E3.5 fertilized female mice or developed in in vitro culture by the parthenogenetic activation of MII oocytes (from C57BL/6 × DBA/2J F1 6-8-week-old-female mice) were lysed in DEPC water, then reversely transcribed with Super Script II Kit (Invitrogen) using the outer 3′ primers for Hprt, Snrpn, Peg1 and Peg3, following the manufacturer's protocol. 1/15 of the product of reverse transcription was used as a template for the 1st PCR (25 μl, Tm = 58 °C, 30 cycle), and 1ìl of the 1st PCR product was used for the 2nd PCR with inner primers (25 μl, Tm = 60 °C, 30 cycle).
Genome methylation assay
Bisulfate-treatment based genomic DNA methylation analysis was conducted following the method described 24. The WT and pES cells of relatively early passage (WT p11, Sp3 p12) that were grown without feeder were harvested and the genomic DNA was extracted using a Genomic DNA Extract Kit (BioDev Biotechnology). Two hundred nanograms of genomic DNA from each sample was digested with HindIII for fragmentation and embedded in low melting point agarose micro-beads (for each sample, 6 beads were formed) for bisulfate treatment to convert the unmethylated C to U. Two-round PCR with treated micro-beads was applied to expand the differentially methylated regions (DMRs) of Gtl2, H19 and Snrpn from the treated genomic DNA. The PCR product was gel purified and digested with restriction enzymes: TaqI (site T/CGA, Takara) for Gtl2 DMR and H19 5′-DMR, and BstUI (site CG/CG, New England Biotechnology) for Snrpn DMR.
Results
Establishment and characterization of mouse pES cell lines
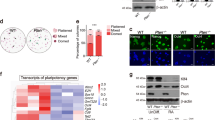
Three mouse diploid pES cell lines were established by super-ovulation of C57BL/6 × DBA/2J or C57BL/6 × CBA F1 female mice, following administration of PMS and HCG and activation of MII oocytes with ionomycin. 6-DMAP was used to suppress the final meiotic reduction division. Therefore, embryos with only one polar body were used to derive the ES cell lines. Two lines (Sp3 and Sp6) were from C57BL/6 × DBA/2J and one line (Sp34) was from C57BL/6 × CBA F1 MII oocytes. For Sp3 and Sp6 lines, 20 activated embryos were used. Sp34 was derived from 15 activated embryos. All three lines formed typical mouse ES cell colonies and the colonies were morphologically indistinguishable from the ES cell colonies that were derived from fertilized blastocysts (Figure 1A). These cells had a high relative nuclear/cytoplasmic ratio and prominent nucleoli. The cultured cells were passed after 2 or 3 days and had a growth rate similar to that of WT mouse ES cells (data not shown). Each of the cell lines was successfully cryopreserved and thawed and has been propagated continuously for more than 50 passages, maintaining their undifferentiated state. Karyotype analysis revealed 40 XX chromosomes, in accordance with their species of origin, when examined at passage 10 (Figure 1B).
Establishment and characterization of mouse parthenogenetic ES cell lines. (A) Typical mouse parthenogenetic ES cell colonies. (B) A representative euploid karyotype observed in mouse parthenogenetic ES cells. (C) Genotypic analysis of Sp3 and Sp6 pES cell lines. (D) AKP positive staining of a pES cell colony. (E) Oct-4 positive staining. (F) Telomerase activity assay. Lane 1: buffer; lane 2: mouse feeder cells; lane 3: telomerase positive control cells; lane 4, 6, 8 and 10: heat-inactivated cell extracts for positive control cells, Sp3, Sp6 and Sp34, respectively; lane 5, 7 and 9: ES cell extracts for Sp3, Sp6 and Sp34, respectively.
Theoretically, pES cells that are derived from chemically activated MII oocytes with the extrusion of the first polar body contain one set of duplicated homologous chromosomes and are homozygous, with perhaps minimal heterozygosity on crossover. To confirm the homozygosity of the established pES cells, their genotype was validated on the basis of the analysis of seven microsatellite markers. The results are summarized in Table 1. Figure 1C illustrates PCR results for the marker D7mit267. The PCR product had two bands for C57BL/6 × DBA/2J F1 female mice. However, a single band was detected from both Sp3 and Sp6 pES cell lines, indicating that the ES cells carried one homologous chromosome from F1 female mice. Similar experiments were also performed with the Sp34 ES cell line and the results were similar to those with Sp3 and Sp6 (data not shown). These observations verify that the genotype of the established pES cell lines is homozygous at the loci examined and the cells are indeed derived from MII oocytes.
Furthermore, expression of Oct-4, AKP and SSEA-1 was examined by immunohistochemistry at passage 11 and after passage 52.. Similar to undifferentiated fES cells, our pES cells expressed high levels of AKP (Figure 1D) and Oct-4 (Figure 1E). SSEA-1 expression was positive (not shown). In addition, telomerase activities in the cell extracts of three lines were measured. All three lines had high levels of tolemerase activity even after passage 50. As shown in Figure 1F, the pattern was similar to that of the positive control (lane 3) provided with the manufacturer's kit. The PCR products seen in lanes 5, 7 and 9 form a ladder that is indicative of the repeated addition of 6 bp to a template by telomerase in the cell extracts obtained from the pES cell lines Sp3, Sp6 and Sp34, respectively. In contrast, there was no detectable telomerase activity in the negative control groups (lane 1 for the buffer and lane 2 for mouse feeder cells; cell extracts in lanes 4, 6, 8 and 10 were inactivated by heat). Moreover, the PCR products from 36 bp internal controls could be detected in all negative control groups, providing evidence that the failure to produce a ladder from products in the negative group was not due to any variations in PCR conditions per se; instead, it was due to a lack of telomerase activity in these samples. On the other hand, the results confirm that the ladder profile of the formed products is reflective of the telomerase activity present in the pES cells. Taken together, the data indicate that our pES cells have the capacity to proliferate and remain undifferentiated for a long period of time in vitro.
In vitro and in vivo differentiation of pES cells
The established pES cell lines were examined for their ability to spontaneously differentiate in vitro. EB formation is an important requirement for validating the in vitro differentiation of ES cells. When our ES cells (Sp3 cells shown representatively) were cultured in suspension, they spontaneously formed sphere-shaped, simple EBs in 2 days (Figure 2A), and formed cavitated EBs after around 4 days (Figure 2B). After about 10 days in suspension culture, the cystic EBs developed one or more balloon-like structures (Figure 2C). Spontaneous rhythmic beating was detected in some areas of the balloon-like structure, suggesting that the cells have the ability to differentiate into cardiomyocyte-like muscle. As with fertilized ES cells, 2 days after plating and attaching the EBs to 0.1% gelatin-coated glass cover slips, they spontaneously differentiated into cell types with specific shapes, including neuron-like cells, muscle-like cells and hepatocyte-like cells (data not shown). Furthermore, expression of markers for the three germ layers was examined by immunofluorescence staining. Positive staining for Map2 (Figure 2D, marker for ectoderm), Desmin (Figure 2E, marker for mesoderm) and GATA4 (Figure 2F, marker for endoderm) was observed, demonstrating that the cells have the capacity to differentiate into the cell types of all three germ layers in vitro. It has been reported that retinoic acid (RA) can induce ES cells to differentiate into neural cells. The pES cells were further examined for their ability to differentiate in vitro in the presence of RA. As expected, in the presence of RA the cells were induced to differentiate into neural lineage cells, as demonstrated by the immunofluorescence staining positive for the neural precursor marker Nestin (Figure 2G), the neuronal marker Tuj-1 (Figure 2H) and the marker for astrocytes GFAP (Figure 2I). This result indicated that the established cell lines could respond to the RA induction of differentiation.
Differentiation of parthenogenetic ES cells in vitro. Cells of all three germ layers were detected in the spontaneous differentiation of pES cells through embryoid bodies (EBs). (A) Simple EBs of pES cells. (B) Cavitated EBs. (C) Cystic EBs. (D) Immunofluorescence staining for ectoderm cells positive for Map2. (E) Mesoderm cells positive for Desmin. (F) Endoderm cells positive for GATA4. (G) Neural precursor cells stained positive for Nestin. (H) Neurons stained positive for Tuj-1. (I) Astrocytes stained positive for GFAP. DAPI was used to label the cell nucleus. Scale bar, 50 μm in all immunofluorescent staining micrographs.
The pluripotency of established mouse pES cell lines was evaluated on the basis of their capacity to elicit teratomatous development after sub-muscular injection at one side of the rear limb in immunodeficient mice (Scid-Beige). All mice developed tumors in 2 weeks. There was intact membrane around the tumors and no evidence of metastatic spread to other tissues was found. Histological examination revealed that all the teratomas contained tissues representative of all three germ layers (Figure 3). The differentiated tissues detected include gut epithelium, glandular epithelium (endoderm), skeletal muscle, smooth muscle, cartilage, fatty tissue (mesoderm), hair follicles, neural tubular epithelium and stratified squamous epithelium (ectoderm). In contrast, only skeletal muscle was found in the contralateral limb where no pES cells were injected. The results demonstrate that pES cells could differentiate into cell types that are derived from ectoderm, mesoderm and endoderm in vivo. Meanwhile, teratomas derived from fertilized ES cells (established in our laboratory) were analyzed in order to compare their differentiation capacity with that of pES cells (data not shown). There was no significant difference in the differentiation of the cell types found between the two teratomas, suggesting that pES cells exhibit a pluripotent differentiation potential that is comparable to fertilized ES cells.
Chimera generation and germline transmssion
To truly demonstrate the developmental pluripotency of established ES cell lines, ES cells from Sp3 and Sp6 lines were injected into blastocyts of ICR mice and the injected embryos were transferred to the uterus of pseudopregnant C57BL/6 × CBA F1 female mice. As summarized in Table 2, the production of chimeric offspring was 67% and 44% for the Sp3 and Sp6 lines, respectively, relative to the number of newborn. Furthermore, germline transmitted mice were produced when chimeric mice were back crossed to WT ICR mice. As shown in Table 2, one female derived from Sp3 ES cells with 80% chimerism produced one male offspring with a gray coat color in her third delivery (1/5) and one female derived from Sp6 ES cells with 10% chimerism produced two male offspring with a black coat color in her fourth (1/6) and fifth (1/10) deliveries. Figure 4A shows chimeric mice obtained by blastocyst injection of Sp3 ES cells, and Figure 4B shows a germline-transmitted mouse from Sp3 ES cells (mouse in gray coat). The result provides a clear demonstration that our pES cells can proliferate and differentiate in vivo.
Expression of imprinted genes in pES cells
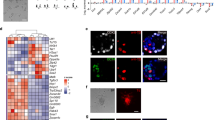
To explore the underlying mechanisms for our pES cells to display normal features of proliferation and differentiation, we determined whether the parental expression of imprinted genes was altered in pES cells. The expression of two paternally expressed genes, Snrpn and U2af1-rs1, and one maternally expressed gene, H19, was first examined by semi-quantitative RT-PCR in an Sp3 ES cell line and a D3 ES cell line (Figure 5A and 5B). As expected, H19 was present in both Sp3 and D3 cell lines, with a higher expression level in the Sp3 line than in D3 ES cells. The absence of the signal in control lanes (without reverse transcriptase in the reaction for the synthesis of first chain cDNA) precludes the possibility of genomic DNA contamination in the reaction. However, the Snrpn and U2af1-rs1 genes were unexpectedly detected in the Sp3 cell line as well as in the D3 cell line. The experiments were repeated three times using D3 and Sp3 ES cells of three different passages and the same result was obtained. In order to exclude the possibility of feeder cell contamination, the presence of the Zfy gene (only present in Y chromosome) in feeder, D3 and Sp3 ES cells was examined. As shown in Figure 5C, the Zfy gene was found in both feeder and D3 cell lines, but was not detectable in Sp3 ES cells. This rules out the possibility that the positive signal for Snrpn and U2af1-rs1 genes found in the Sp3 cell line was due to the contamination of feeder cells.
Analysis of expression of imprinted genes in pES cells. (A) Semi-quantitative RT-PCR analysis of three imprinted genes was performed with D3 and Sp3 ES cells. Feeder cells were removed from D3 and Sp3 ES cells for 6, 7 and 8 times; the cells from different passages were labeled as D1, D2, D3 and S1, S2, S3, respectively. Hprt was used for loading control. (B) Relative expression level of the imprinted genes. The density of specific bands in RT-PCR data from (A) was measured by densitometer and normalized by Hprt. The expression level of D3 was designated as 1.0 and the ratio of Sp3 to D3 was presented. (C) Genomic PCR of the Zfy gene. β-Actin was used for loading control. (D) Determination of the expression level of imprinted genes in Sp3 and D3 ES cell lines by real time RT-PCR. The tests were performed in three independent experiments. The results were presented as a ratio between Sp3 and D3 (as 1.0) mRNA levels with standard variation. (E) Comparison of the expression levels of paternally expressed imprinted genes between pES cells (Sp3) and wild-type (WT) ES cells (as 1.0) at different passages (10 and 20). (F) The activation of the paternally expressed imprinted genes Snrpn, Mest and Peg3 in parthenogenetic blastocysts. Hprt was used for loading control. P and WT stand for parthenogenetic and wild-type blastocysts, respectively.
Furthermore, to determine whether the activation of paternally expressed imprinted genes found in our cell lines can be applied to other imprinted genes, expression of six more developmentally related imprinted genes, Peg3, Zfp127, Ndn, Impact, Dlk1 and Mest, was examined by quantitative real time RT-PCR. The primers used are shown in Supplementary information, Table 2. As shown in Figure 5D, expression of all six paternally expressed imprinted genes was found in Sp3(p21) ES cells, although the expression levels of Dlk1 and Mest in Sp3 cells were much lower than in D3(p21) cells. Expression of these genes in Sp6 and Sp34 was also detected (see Supplementary information, Figure S1). These observations suggest that some of the paternally expressed imprinted genes were activated in our pES cell lines and this may contribute to their developmental competence.
We were interested in how these paternally expressed imprinted genes are activated in pES cells. It has been reported that the deregulation in expression of imprinted genes in ES cells is frequently associated with in vitro manipulation 25. To analyze the effect of in vitro culture on expression of imprinting genes, expression of paternally expressed imprinted genes at p10 and p20 of Sp3 ES cells was analyzed in comparison with expression of the genes in a WT ES cell line (WT ES cell line) at the same passage. The WT ES cell line was established in our laboratory from mice that have the same genetic background as Sp3 and Sp6. As shown in Figure 5E, expression of all six imprinted genes was detectable but was much lower, except for Impact, in Sp3 cells than in WT ES cells at p10. However, the ratio of expression between Sp3 and WT ES cells for these genes increased substantially after the cells were passaged for another 10 times (at p20). This finding suggests that culturing pES cells influences expression of paternally expressed imprinted genes. On the other hand, it is unclear when the activation of paternal gene expression commences. Does it initiate after derivation and culture of ES cells from the blastocyst or does it exist before the derivation? To answer this question, the transcription of three paternally expressed imprinted genes, Snrpn, Mest and Peg3, in the parthenogenetically activated and fertilized blastocysts was analyzed using nest-PCR. To our surprise, expression of these three imprinted genes was found not only in fertilized blastocysts but also in parthenogenetically activated blastocysts (Figure 5F), indicating that the activation of some paternally expressed imprinted genes initiates before the derivation of pES cells. Therefore, both in vitro manipulation of embryos and extended culture of pES cells play a role in deregulating the imprinting status detected in this study.
Activating paternally expressed imprinted genes is associated with altered methylation patterns
DNA methylation is an important mechanism for genomic imprinting, and epigenetic instability has been reported in DMRs of some imprinted genes 25. Therefore, we examined whether the activation of the imprinted genes in our study was associated with altered methylation of the related DMRs. To this end, bisulfate nest PCR and restricted fragment length polymorphism techniques were applied to analyze the methylation of Dlk1-Gtl2, H19-Igf2 and Snrpn DMRs. It is known that Gtl2 and H19 DMRs should be completely unmethylated in order to silence paternally expressed Dlk1 and Igf2 genes, while the Snrpn DMR should be entirely methylated in order to suppress transcription from the maternal genome 24. As was to be expected, we found that about half of the PCR products were digested by methylation-sensitive restriction enzymes for Gtl2 and Snrpn DMRs in the WT ES cells, suggesting that both paternal and maternal genomes are present and half the genomic DNA is methylated. However, it seems that the DMR for H19 was heavily methylated, resulting in the majority of DMRs being digested (Figure 6A). Interestingly, partial methylation was also found at Gtl2 and H19 DMRs of Sp3 ES cells. In addition, incomplete methylation at the Snrpn DMR was detected in these cells. The results demonstrate that the methylation patterns at DMRs were altered in our pES cells. The contamination of feeder genomic DNA was excluded by the absence of Zfy genomic DNA in an Sp3 genomic DNA preparation (see Supplementary information, Figure S2). To further confirm the results from Sp3 pES cells, we derived additional pES cell lines without feeder layers (Sp1 and Sp2). The cells of Sp1 at passage 5 and the cells of Sp2 at passage 6 were used for methylation analysis. Similar results were obtained (Figure 6B), confirming that the altered methylation found in Sp3 ES cells is not a result of feeder contamination, but is a general phenomenon in pES cell lines.
Overall methylation status of the DMRs in mouse pES cells. The bisulfite-treated DNA was amplified by PCR and digested by enzymes that cut only if the site was methylated. (A) The methylation pattern of wild-type (WT) ES cells and Sp3 pES cells at Gtl2, H19 and Snrpn DMRs. (B) The methylation pattern of two additional pES cell lines, Sp1 and Sp2. The enzyme used for DNA digestion is shown and - or + indicates the undigested or digested DNA. Sizes of digested fragments are indicated on the right.
Discussion
Parthenogenesis is the process by which an egg is induced to develop without the contribution of sperm 26. Some lower organisms routinely reproduce in this manner 27. In mammals, an egg can be activated artificially. However, the embryos cannot survive to term. Mouse parthenogenetic embryos die by day 10 of development 28. It has been reported that parthenotes fail to develop a trophectoderm and primitive endoderm-extra embryonic tissues 26. The reason for this failure in mammalian development is believed to be the disruption of genomic imprinting 8. Obviously, both parental genomes are required for the successful development of mammals 12. Although the stem cell lines from mouse homozygous embryos have been established and studied since 1983, there are still controversies regarding the pluripotency of pES cells 29. Park et al. 11 found that EBs derived from pES cells were retarded in growth and showed restricted differentiation compared to their fertilized counterpart. It was also reported that pES cells showed a restricted tissue distribution in chimeras with normal host cells. However, the classic phenotype of growth retardation was not seen in the chimeras 8. Contrary to the above finding, Lin et al. 7 demonstrated that homozygous mouse ES cells derived from MII oocytes have multilineage differentiation potential. However, the study did not demonstrate whether these homogzygous stem cells could produce chimeras and make germline transmissions, which is considered to be the most robust demonstration for pluripotency of any established ES cell lines. Furthermore, a non-human primate pES cell line was established recently. It was maintained in vitro in an undifferentiated state for extended periods of time and differentiated into dopaminergic and serotonergic neurons, contractile cardiomyocyte-like cells, smooth muscle, ciliated epithelia and adipocytes. When the cells were injected into the peritoneal cavity of immunocompromised severe combined immunodeficient mice, derivatives of all three germ layers were observed 6. In this study, the pluripotent differentiation capacities of mouse pES cells were demonstrated by their ability to produce multi-lineages of cells derived from all three germ layers both in vitro and in vivo, and more importantly by their ability to produce chimeras and make germline transmissions that are comparable to those of fertilized mouse ES cells. Moreover, these cells remained undifferentiated after more than 50 passages in culture. This characteristic makes it practical to obtain as many cells as needed.
The recent birth of a parthenogenetic mouse using non-growing oocyte with a 13-kilobase deletion in the H19 gene as donor provides direct evidence that genomic imprinting is a major barrier to parthenogenetic development 30. The mechanisms of genomic imprinting are largely unknown at present, although substantial progress has been made in improving our understanding of genomic imprinting over the past few years. It is generally believed that germline-specific epigenetic modifications, including allele-specific DNA methylation and chromatin structural remodeling, are possible imprinting mechanisms. pES cells have two sets of maternal genomes, but lack the paternal genome. If parental allele-specific expressions of imprinted genes were maintained in pES cells, their proliferation or differentiation capacities would be expected to be abnormal. However, we did not find any differences regarding growth rate or differentiation potential between parthenogenetic mouse ES cells and fertilized ES cells. Therefore, expression of nine known imprinted genes in pES cells was analyzed in comparison with their expression in D3 fertilized mouse ES cells. These nine genes were chosen because expression of these genes is developmentally regulated and they play an important role in embryonic or disease development. For example, H19 is activated during ES cell differentiation in vitro and at the time of implantation in the developing embryo 31. In contrast, our previous study showed that SNRPN was significantly downregulated during hES cell differentiation in vitro 32. In addition, it has been suggested that PEG3 functions as a tumor suppressor 4. The necdin gene NDN is known to be maternally imprinted and expressed only from the paternal allele. Its deficiency is involved in the pathogenesis of the neurodevelopmental disorder Prader-Willi syndrome 33. In this study, as expected, the maternally expressed imprinted gene, H19, was expressed in the Sp3 cell line at a higher level than in the D3 cell line. The paternally expressed imprinted genes were also found to be expressed in the Sp3 cell line and in the D3 cell line. This observation is consistent with the report from Szabo and Mann, who found that Snrpn was expressed in pES derived EBs 34. In addition, the disrupted expression of U2af1-rs1 was also observed in maternal alleles in parthenogenetic fetuses 18. However, the disrupted expression of Peg3, Impact, Zfp127, Ndn, Dlk1 and Mest in pES cells has not been reported. These genes are critical for regulating cell proliferation, differentiation and cell death during early mammalian development. For example, the expression pattern of Ndn is involved with the etiology of PWS 33. Mice with a deletion involving both Snrpn and the putative PWS-Imprinting Center lack expression of the genes Zfp127 and Ndn and manifest several phenotypes common to PWS infants 35. In addition, it was reported that abnormal maternal behavior and growth retardation is associated with loss of the imprinted gene Mest 36. Therefore, the disrupted expression of these maternally imprinted genes may explain why our pES cells exhibit normal growth and differentiation properties. In addition to the genes mentioned above, there are other reports about the disruption of specific expression of imprinted genes in uniparental fetuses and embryonic stem cells 37. However, the mechanisms for the disruption remain largely unknown. One possibility is that paternal expression is necessary for establishing and/or maintaining the maternal repression of some imprinted genes. An alternative is that there is an underlying dose-compensation mechanism to establish and/or maintain parental allele-specific expression of imprinted genes. In addition, there may be some adaptation to culture conditions during the derivation and amplification of pES cells, involving certain changes in the epigenetic modification of genes. Our findings provide experimental evidence for the influence of in vitro culture on the epigenetic stability and genomic imprinting in pES cells. Furthermore, we show here that deregulation of imprinting is initiated before derivation of pES cells and extended culture also plays a role in the activation of expression of paternal genes. More importantly, we demonstrate that the activation of paternally expressed imprinted genes in pES cells is associated with an alteration in methylation patterns of related DMRs. It is worthy to note that the methylation pattern of these DMRs is consistent with the mRNA level of related imprinted genes. For example, the methylation level of Gtl2 DMRs in pES cells was much lower than in WT ES cells (Figure 5A, left column). In line with this, expression of Dlk1 in p10 pES cells was about half that of the WT ES cells (Figure 4D and 4E). Taken together, our observations indicate that the disruption of genomic methylation might be a premise for the parthenogenetically activated oocytes to develop into the blastocyst stage and thus allow successful pES cell derivation. The above results also implicate that the presence of the paternal genome is important for the imprinting stability of the maternal genome. Obviously, a detailed and systematic analysis is required to fully explore the relationship between the molecular regulation of expression of imprinted genes and the phenotypic features of both biparental and uniparental fetuses and ES cells. Further investigation of the molecular mechanisms underlying the disruption of imprinted status in parthenogenetic embryos and/or ES cells would provide greater insight into the characteristics of imprinted genes.
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
References
Tesar PJ . Derivation of germ-line-competent embryonic stem cell lines from preblastocyst mouse embryos. Proc Natl Acad Sci USA 2005; 102:8239–8244.
Smith AG . Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol 2001; 17:435–462.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282:1145–1147.
Dowdy SC, Gostout BS, Shridhar V, et al. Biallelic methylation and silencing of paternally expressed gene 3 (PEG3) in gynecologic cancer cell lines. Gynecol Oncol 2005; 99:126–134.
Kim K, Lerou P, Yabuuchi A, et al. Histocompatible embryonic stem cells by parthenogenesis. Science (New York, NY) 2007; 315:482–486.
Vrana KE, Hipp JD, Goss AM, et al. Nonhuman primate parthenogenetic stem cells. Proc Natl Acad Sci USA 2003; 100 Suppl 1:11911–11916.
Lin H, Lei J, Wininger D, et al. Multilineage potential of homozygous stem cells derived from metaphase II oocytes. Stem Cells 2003; 21:152–161.
Allen ND, Barton SC, Hilton K, Norris ML, Surani MA . A functional analysis of imprinting in parthenogenetic embryonic stem cells. Development 1994; 120:1473–1482.
Newman-Smith ED, Werb Z . Stem cell defects in parthenogenetic peri-implantation embryos. Development 1995; 121:2069–2077.
Jagerbauer EM, Fraser A, Herbst EW, Kothary R, Fundele R . Parthenogenetic stem cells in postnatal mouse chimeras. Development 1992; 116:95–102.
Park JI, Yoshida I, Tada T, et al. Differentiative potential of a mouse parthenogenetic embryonic stem cell line revealed by embryoid body formation in vitro. Jpn J Vet Res 1998; 46:19–28.
McGrath J, Solter D . Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 1984; 37:179–183.
Haig D . Genomic imprinting and kinship: how good is the evidence? Annu Rev Genet 2004; 38:553–585.
Delaval K, Feil R . Epigenetic regulation of mammalian genomic imprinting. Curr Opin Genet Dev 2004; 14:188–195.
Mitalipov SM . Genomic imprinting in primate embryos and embryonic stem cells. Reprod Fertil Dev 2006; 18:817–821.
Lopes S, Lewis A, Hajkova P, et al. Epigenetic modifications in an imprinting cluster are controlled by a hierarchy of DMRs suggesting long-range chromatin interactions. Hum Mol Genet 2003; 12:295–305.
da Rocha ST, Ferguson-Smith AC . Genomic imprinting. Curr Biol 2004; 14:R646–R649.
Sotomaru Y, Kawase Y, Ueda T, et al. Disruption of imprinted expression of U2afbp-rs/U2af1-rs1 gene in mouse parthenogenetic fetuses. J Biol Chem 2001; 276:26694–26698.
Dean W, Bowden L, Aitchison A, et al. Altered imprinted gene methylation and expression in completely ES cell-derived mouse fetuses: association with aberrant phenotypes. Development 1998; 125:2273–2282.
Mitalipov SM, Nusser KD, Wolf DP . Parthenogenetic activation of rhesus monkey oocytes and reconstructed embryos. Biol Reprod 2001; 65:253–259.
Liu L, Ju JC, Yang X . Parthenogenetic development and protein patterns of newly matured bovine oocytes after chemical activation. Mol Reprod Dev 1998; 49:298–307.
Xu HM, Liao B, Zhang QJ, et al. Wwp2, an E3 ubiquitin ligase that targets transcription factor Oct-4 for ubiquitination. J Biol Chem 2004:23495–23503.
Keller GM . In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 1995; 7:862–869.
Li JY, Lees-Murdock DJ, Xu GL, Walsh CP . Timing of establishment of paternal methylation imprints in the mouse. Genomics 2004; 84:952–960.
Schumacher A, Doerfler W . Influence of in vitro manipulation on the stability of methylation patterns in the Snurf/Snrpn-imprinting region in mouse embryonic stem cells. Nucleic Acids Res 2004; 32:1566–1576.
Surani MA, Barton SC . Development of gynogenetic eggs in the mouse: implications for parthenogenetic embryos. Science (New York, NY) 1983; 222:1034–1036.
Kim K, Lerou P, Yabuuchi A, et al. Histocompatible embryonic stem cells by parthenogenesis. Science 2007; 315:482–486.
Barton SC, Surani MA, Norris ML . Role of paternal and maternal genomes in mouse development. Nature 1984; 311:374–376.
Kaufman MH, Robertson EJ, Handyside AH, Evans MJ . Establishment of pluripotential cell lines from haploid mouse embryos. J Embryol Exp Morphol 1983; 73:249–261.
Kono T, Obata Y, Wu Q, et al. Birth of parthenogenetic mice that can develop to adulthood. Nature 2004; 428:860–864.
Poirier F, Chan CT, Timmons PM, et al. The murine H19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development 1991; 113:1105–1114.
Sun BW, Yang AC, Feng Y, et al. Temporal and parental-specific expression of imprinted genes in a newly derived Chinese human embryonic stem cell line and embryoid bodies. Hum Mol Genet 2006; 15:65–75.
Andrieu D, Watrin F, Niinobe M, et al. Expression of the Prader-Willi gene Necdin during mouse nervous system development correlates with neuronal differentiation and p75NTR expression. Gene Expr Patterns 2003; 3:761–765.
Szabo P, Mann JR . Expression and methylation of imprinted genes during in vitro differentiation of mouse parthenogenetic and androgenetic embryonic stem cell lines. Development 1994; 120:1651–1660.
Yang T, Adamson TE, Resnick JL, et al. A mouse model for Prader-Willi syndrome imprinting-centre mutations. Nat Genet 1998; 19:25–31.
Lefebvre L, Viville S, Barton SC, et al. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet 1998; 20:163–169.
Sotomaru Y, Katsuzawa Y, Hatada I, et al. Unregulated expression of the imprinted genes H19 and Igf2r in mouse uniparental fetuses. J Biol Chem 2002; 277:12474–12478.
Acknowledgements
The study was supported by grants from the Project of Shanghai Science & Technology Developmental Foundation (No. 04DZ14006), Chinese Academy of Sciences and Shanghai Jiao Tong University School of Medicine.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jiang, H., Sun, B., Wang, W. et al. Activation of paternally expressed imprinted genes in newly derived germline-competent mouse parthenogenetic embryonic stem cell lines. Cell Res 17, 792–803 (2007). https://doi.org/10.1038/cr.2007.70
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2007.70
Keywords
This article is cited by
-
Ascorbic acid improves pluripotency of human parthenogenetic embryonic stem cells through modifying imprinted gene expression in the Dlk1-Dio3 region
Stem Cell Research & Therapy (2015)
-
High-throughput sequencing reveals the disruption of methylation of imprinted gene in induced pluripotent stem cells
Cell Research (2014)
-
Derivation of androgenetic embryonic stem cells from m-carboxycinnamic acid bishydroxamide (CBHA) treated androgenetic embryos
Chinese Science Bulletin (2013)
-
Derivation of haploid embryonic stem cells from mouse embryos
Nature (2011)
-
Quantitative proteomics analysis of parthenogenetically induced pluripotent stem cells
Protein & Cell (2011)