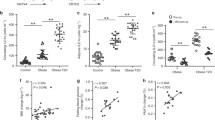
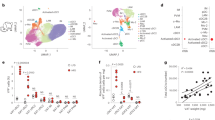
Abstract
Metaflammation is responsible for several metabolic syndromes, such as type 2 diabetes. However, the mechanisms by which metabolic disorders trigger metaflammation remain unclear. We identified a cell type-specific downregulation of CD1d expression in M2 macrophages during the progression of obesity prior to the onset of inflammation in visceral adipose tissues. A reduction in CD1d expression influenced the ability of M2 macrophages to present antigens and caused a change in antigen-presenting cells from M2 macrophages to M1 macrophages. With CD1d conditional knockout (KO) mice, we further demonstrated that natural killer T (NKT) cell activation by M2 macrophages inhibited metaflammation and insulin resistance by promoting Th2 responses and M2 polarization in visceral adipose tissues of obese mice, whereas NKT cell activation by M1 macrophages exacerbated metaflammation and insulin resistance by promoting Th1 responses and inhibiting M2 polarization. Our results suggest that an M2-specific reduction of CD1d is an initiating event that switches NKT cell-mediated immune responses and disrupts the immune balance in visceral adipose tissues in obese mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kammoun HL, Kraakman MJ, Febbraio MA. Adipose tissue inflammation in glucose metabolism. Rev Endocr Metab Disord 2014; 15: 31–44.
Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011; 121: 2111–2117.
Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72: 219–246.
Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009; 58: 2574–2582.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Xu HY, Barnes GT, Yang Q, Tan Q, Yang DS, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821–1830.
Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 2013; 210: 535–549.
Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011; 332: 243–247.
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15: 914–920.
McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity 2014; 41: 36–48.
Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med 2011; 208: 1179–1188.
Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat Immunol 2015; 16: 85–95.
Mathews S, Feng D, Maricic I, Ju C, Kumar V, Gao B. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Cell Mol Immunol 2016; 13: 206–216.
Bandyopadhyay K, Marrero I, Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell Mol Immunol 2016; 13: 337–346.
Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol 2003; 171: 1637–1641.
Wingender G, Rogers P, Batzer G, Lee MS, Bai D, Pei B et al. Invariant NKT cells are required for airway inflammation induced by environmental antigens. J Exp Med 2011; 208: 1151–1162.
Wu L, Van Kaer L. Contribution of lipid-reactive natural killer T cells to obesity-associated inflammation and insulin resistance. Adipocyte 2013; 2: 12–16.
Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest 2012; 122: 3343–3354.
Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 2012; 37: 574–587.
Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci USA 2012; 109: E1143–E1152.
Ji Y, Sun S, Xia S, Yang L, Li X, Qi L. Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J Biol Chem 2012; 287: 24378–24386.
Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS One 2012; 7: e30568.
Bai L, Constantinides MG, Thomas SY, Reboulet R, Meng F, Koentgen F et al. Distinct APCs explain the cytokine bias of alpha-galactosylceramide variants in vivo. J Immunol 2012; 188: 3053–3061.
Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol 2013; 25: 161–167.
Sullivan BA, Nagarajan NA, Wingender G, Wang J, Scott I, Tsuji M et al. Mechanisms for glycolipid antigen-driven cytokine polarization by Valpha14i NKT cells. J Immunol 2010; 184: 141–153.
Scanlon ST, Thomas SY, Ferreira CM, Bai L, Krausz T, Savage PB et al. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J Exp Med 2011; 208: 2113–2124.
Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 2005; 3: e113.
Colgan SP, Pitman RS, Nagaishi T, Mizoguchi A, Mizoguchi E, Mayer LF et al. Intestinal heat shock protein 110 regulates expression of CD1d on intestinal epithelial cells. J Clin Invest 2003; 112: 745–754.
Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature 2014; 509: 497–502.
Tatsumi T, Takehara T, Yamaguchi S, Sasakawa A, Yamamoto M, Fujita Y et al. Decreased expressions of CD1d molecule on liver dendritic cells in subcutaneous tumor bearing mice. J Hepatol 2008; 49: 779–786.
Yuan W, Dasgupta A, Cresswell P. Herpes simplex virus evades natural killer T cell recognition by suppressing CD1d recycling. Nat Immunol 2006; 7: 835–842.
Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest 2005; 115: 1369–1378.
Bai L, Sagiv Y, Liu Y, Freigang S, Yu KO, Teyton L et al. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen alphaGalCer. Proc Natl Acad Sci USA 2009; 106: 10254–10259.
Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med 2011; 208: 1163–1177.
Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol 2003; 4: 1230–1237.
Goto-Inoue N, Yamada K, Inagaki A, Furuichi Y, Ogino S, Manabe Y et al. Lipidomics analysis revealed the phospholipid compositional changes in muscle by chronic exercise and high-fat diet. Sci Rep 2013; 3: 3267.
Pietilainen KH, Rog T, Seppanen-Laakso T, Virtue S, Gopalacharyulu P, Tang J et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Biol 2011; 9: e1000623.
Jove M, Moreno-Navarrete JM, Pamplona R, Ricart W, Portero-Otin M, Fernandez-Real JM. Human omental and subcutaneous adipose tissue exhibit specific lipidomic signatures. FASEB J 2014; 28: 1071–1081.
Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol 2007; 178: 2706–2713.
Nakamura T, Sonoda KH, Faunce DE, Gumperz J, Yamamura T, Miyake S et al. CD4+ NKT cells, but not conventional CD4+ T cells, are required to generate efferent CD8+ T regulatory cells following antigen inoculation in an immune-privileged site. J Immunol 2003; 171: 1266–1271.
Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol 1999; 163: 4647–4650.
Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med 2003; 198: 267–279.
Lee BC, Kim MS, Pae M, Yamamoto Y, Eberle D, Shimada T et al. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab 2016; 23: 685–698.
Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol 2015; 16: 376–385.
O’Rourke RW, Meyer KA, Neeley CK, Gaston GD, Sekhri P, Szumowski M et al. Systemic NK cell ablation attenuates intra-abdominal adipose tissue macrophage infiltration in murine obesity. Obesity 2014; 22: 2109–2114.
Huh JY, Kim JI, Park YJ, Hwang IJ, Lee YS, Sohn JH et al. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol Cell Biol 2013; 33: 328–339.
Martin-Murphy BV, You Q, Wang H, De La Houssaye BA, Reilly TP, Friedman JE et al. Mice lacking natural killer T cells are more susceptible to metabolic alterations following high fat diet feeding. PLoS One 2014; 9: e80949.
Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J 2010; 4: 232–241.
Escobedo G, Lopez-Ortiz E, Torres-Castro I. Gut microbiota as a key player in triggering obesity, systemic inflammation and insulin resistance. Rev Invest Clin 2014; 66: 450–459.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184.
Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 2014; 41: 21–35.
Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014; 14: 392–404.
Acknowledgements
We thank the NIH Tetramer Core Facility for providing us with the CD1d-PBS57 tetramer. This work was supported by the Major State Basic Research Development Program of China (973 Program) 2013CB944902, the National Natural Science Foundation of China 91542203 and 31470859, the Strategic Priority Research Program of the Chinese Academy of Sciences XDA12030201, the Fundamental Research Funds for the Central Universities and Users with Potential 2015HSC-UP018.
Author information
Authors and Affiliations
Contributions
HZ and RX contributed equally to the paper. HZ, RX, SZ, SF and ZC performed the experiments. RZ and ZT discussed the experiments. HZ, RX and LB designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, H., Xue, R., Zhu, S. et al. M2-specific reduction of CD1d switches NKT cell-mediated immune responses and triggers metaflammation in adipose tissue. Cell Mol Immunol 15, 506–517 (2018). https://doi.org/10.1038/cmi.2017.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2017.11
Keywords
This article is cited by
-
EGFR tyrosine kinase activity and Rab GTPases coordinate EGFR trafficking to regulate macrophage activation in sepsis
Cell Death & Disease (2022)
-
Metabolic control of T-cell immunity via epigenetic mechanisms
Cellular & Molecular Immunology (2018)
-
Tissue-specific functions of invariant natural killer T cells
Nature Reviews Immunology (2018)