Abstract
Polybrominated diphenyl ethers (PBDEs) are ubiquitous environmental pollutants that accumulate to high levels in human populations that are subject to occupational or regional industry exposure. PBDEs have been shown to affect human neuronal, endocrine and reproductive systems, but their effect on the immune system is not well understood. In this study, experimental adult mice were intragastrically administered 2,2′,3,3′,4,4′,5,5′,6,6′-decabromodiphenyl ether (BDE-209) at doses of 8, 80 or 800 mg/kg of body weight (bw) at 2-day intervals. Our results showed that continuous exposure to BDE-209 resulted in high levels of BDE-209 in the plasma that approached the levels found in people who work in professions with high risks of PDBE exposure. Reduced leukocytes, decreased cytokine (IFN-γ, IL-2 and TNF-α) production and lower CD8 T-cell proliferation were observed in the mice exposed to BDE-209. Additionally, mice with long-term BDE-209 exposure had lower numbers of antigen-specific CD8 T cells after immunization with recombinant Listeria monocytogenes expressing ovalbumin (rLm-OVA) and the OVA-specific CD8 T cells had reduced functionality. Taken together, our study demonstrates that continuous BDE-209 exposure causes adverse effects on the number and functionality of immune cells in adult mice.
Similar content being viewed by others
Introduction
Polybrominated diphenyl ethers (PBDEs) are classical brominated flame retardants with excellent thermal stability that have been widely used in different types of industrial and consumer products.1 PBDEs have become ubiquitous environmental pollutants that can be detected in the outdoor and indoor air, dust, water, soil, birds and fish, as well as in human adipose tissue, cord blood and breast milk.2,3,4 Human are exposed to PBDEs via external (e.g., air and dust) and internal routes (e.g., breast milk and cord blood).5 A significantly higher body burden of PBDEs is observed in the US population than in Japan and EU, and recent studies show increasing levels of PBDEs in rapidly developing countries such as China and India.2,6,7,8 Furthermore, the level of 2,2′,3,3′,4,4′,5,5′,6,6′-decabromodiphenyl ether (BDE-209) is extremely high in certain populations that are subject to occupational or regional industry exposure.9
There are 209 congeners among the PBDEs with three commercialized genres known as penta-, octa- and deca-BDE.10 Penta-BDE and octa-BDE are no longer produced or commercialized in most countries.11 BDE-209 is a fully brominated high-molecular-weight deca-BDE and is currently the most widely used PDBE due to its relatively low toxicity. However, high levels of environmental and biological accumulation of BDE-209 have been observed, including more than 28 000 ng/g in mud carp (Cirrhinus molitorella) and 3100 ng/g of lipids in serum samples from the inhabitants of Guiyu, an electronic waste dismantling region of South China.9,12,13,14 Recent studies indicate that in both the environment and organisms, BDE-209 can be debrominated to lower congeners, which have higher risks of bioaccumulation and toxicity.15,16
Many studies have demonstrated that exposure to PBDEs can cause a variety of toxicities in animals and humans, e.g., developmental neurotoxicity, endocrine disturbances and reproductive toxicity.17,18,19 The effect of PBDE exposure on the immune system has also been studied, but these investigations have been limited to in vitro studies, short-term in vivo animal exposure studies and human epidemiology studies of relatively small populations.20,21 In a longitudinal cohort study of 33 children, Leijs et al. found that serum ΣPBDE levels were inversely correlated with the lymphocyte count,21 whereas another study showed that sub-acute exposure (28 days) to deca-BDE did not affect the spleen or blood immune cells in Wistar rats.22 Fair et al. reported that mitogen-stimulated T-cell proliferation was increased after oral gavage with DE-71 (a commercial penta–BDE mixture) for 28 days in adult mice,23 whereas suppressed T lymphocyte multiplication was observed following BDE-209 exposure in rats during pregnancy and lactation.20 The effects of long-term exposure to high levels of PBDEs that more closely mimics human exposure in highly contaminated regions and occupations have not been examined.
In this study, we continuously exposed adult mice to BDE-209 by intragastric administration over 7 months. This exposure model resulted in a high burden of BDE-209 in the plasma that approached the levels observed in people who work in professions with high exposure to PDBEs. The effect of BDE-209 exposure on the quantity and functionality of immune cells was investigated. Our data showed that continuous BDE-209 exposure resulted in fewer leukocytes, reduced the functionality and proliferative capacity of CD8 T cells and impaired the antigen-specific CD8 T-cell response to infection.
Materials and methods
Mice
Female C57BL/6 (CD45.2+) mice (6–8 weeks of age) were obtained from the Shanghai Laboratory Animal Center, Chinese Academy of Science, and housed under specific pathogen-free conditions. All experiments were conducted in compliance with the animal care and use committee guidelines of the Shanghai Jiaotong University School of Medicine (Shanghai, China).
BDE-209 exposure
BDE-209 with a purity of 98% was obtained from Wako Pure Chemical Industries, Ltd (Chuo-ku, Osaka, Japan). The experimental mice were intragastrically administered BDE-209 at doses of 8, 80 or 800 mg/kg body weight (bw, dissolved in 400 µl corn oil) every 2 days, and the control mice were administered 400 µl of corn oil every 2 days.
Hematological examination
Peripheral blood (150 µl/mouse) was harvested from the retro-orbital venous plexus and collected in RPMI 1640 containing 2% EDTA. Hematological examinations were performed using a Coulter AcT Diff hematology analyzer (Beckman Coulter, Inc., Brea, CA, USA) or a HEMAVET 950 multispecies hematology instrument (DREW Scientific, Inc., Dallas, TX, USA).
Infection with recombinant Listeria monocytogenes expressing ovalbumin (rLm-OVA)
Control and experimental mice that were exposed to BDE-209 (800 mg/kg bw at 2-day intervals) for 10 months were immunized intravenously with a dose of 5×104 CFU of rLm-OVA. At day 7 post-infection, OVA257–264-specific CD8 T cells were detected with MHC/peptide tetramers or intracellular cytokine staining.
In vitro stimulation
To evaluate cytokine production by the total population of CD8 T cells, peripheral blood mononuclear cells (PBMCs) or splenocytes were cultured with PMA (50 ng/ml), ionomycin (500 ng/ml) and Golgistop (1.0 µl/ml) for 5 h. For antigen-specific CD8 T-cell detection, PBMCs were stimulated with OVA257–264 peptide at 1.0 µg/ml for 3 h, and Golgistop (1.0 µl/ml) was added for 2 h before analysis. To study the proliferation of CD8 T cells, PBMCs were labeled with CFSE (5 µM) and stimulated with pre-bound anti-CD3e (2 µg/ml) plus soluble anti-CD28 (5 µg/ml) for 24, 48 or 72 h. The splenic CD8 T cells, which were purified from splenocytes by sorting on a FACS Aria II (BD Biosciences, San Jose, CA, USA) based on the surface expression of CD3 and CD8 (CD3+CD8+), were labeled with CFSE (5 µM) and then stimulated with anti-CD3 and anti-CD28 mAbs coupled to magnetic beads at a cell-bead ratio of 4∶1 for 72 h.
Flow cytometry
Surface staining and intracellular cytokine staining were performed as previously described.24 Briefly, surface staining was performed in 100 µl PBS with 3% (v/v) FBS and different antibody cocktails for surface markers (anti-CD3e, anti-CD4, anti-CD8a, anti-CD44, anti-CD62L, anti-B220, anti-CD11b, anti-Ly6C, anti-Ly6G and anti-CD11c) at room temperature for 30 min. Post-staining for surface markers (anti-CD3e, anti-CD4, anti-CD8a and anti-CD44), the intracellular cytokines were stained using intracellular staining kits from BD Biosciences. The cells were incubated with anti-IFN-γ, anti-IL-2 and anti-TNF-α for 40 min at room temperature. OVA-specific CD8 T cells were also quantified by direct staining with H2-Kb/OVA257–264 (MHC/peptide) tetramers. All samples were analyzed on a FACS Canto II flow cytometer (BD Biosciences), and all fluorochrome-conjugated mAbs were purchased from BD Pharmingen (San Jose, CA, USA).
Determination of BDE-209 concentration
The concentration of BDE-209 in mouse plasma was determined as previously described with some modifications.25,26 Briefly, 25 µl blood samples were added to 30 ml n-hexane spiked with the surrogate and internal standard 13C-BDE-209 (Cambridge Isotope Laboratories, Inc., Tewksbury, MA, USA), and 10 ml of concentrated sulfuric acid were added. The mixtures were violently shaken to remove lipids, and the organic phases were further purified using a multilayer silica–alumina column. The concentration of BDE-209 was quantified using an HP 6890N gas chromatograph with a DB-5MS capillary column (15 m×0.25 mm×0.10 µm; J & W Scientific, Inc., Folsom CA, USA) coupled to an HP 5975 mass spectrometer under the negative chemical ionization mode.
Statistical analyses
All experiments were presented as the mean±standard error. The statistical analysis was performed using an unpaired Student's t-test, one-way ANOVA or two-way repeated-measures ANOVA. P-values<0.05 were considered to be statistically significant.
Results
Continuous BDE-209 exposure results in a high burden of BDE-209 in the plasma and reduced leukocytes in peripheral blood
To examine the effect of BDE-209 exposure on the immune cells, we exposed adult B6 mice to BDE-209 continuously by intragastric administration. The BDE-209 accumulation in the plasma and the bw gain were monitored over a period of 7 months in the control mice and the experimental mice that received a dose of 800 mg/kg bw at 2-day intervals. The level of BDE-209 in the plasma of the BDE-209-exposed mice increased in the first month and reached a steady-state level of approximately 200–300 ng/ml or 40 000–60 000 ng/g lipid (1 ml plasma contains ∼0.005 g lipid) that was maintained throughout the rest of the experiment (Figure 1a). The body weight of the BDE-209 exposed mice was significantly lower than of controls after exposure for more than 5 months, and no significant differences were observed between those two groups of mice during months 1 through 3 (Figure 1b).
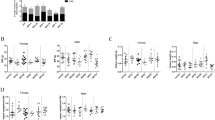
Long-term BDE-209 exposure resulted in a high burden of BDE-209 in the plasma, retarded growth and reduced leukocytes in the peripheral blood. The BDE-209-exposed mice were intragastrically administered BDE-209 at a dose of 800 mg/kg bw at 2-day intervals. (a) The BDE-209 concentration in the plasma was determined by GC–MS under the NCI mode at day 7 and months 1, 2, 5 and 7 (n=10 mice per group, *P<0.05 and ***P<0.001 compared to the control group, two-way repeated-measures ANOVA with Bonferroni post-tests, the means±s.d. are depicted). (b) Body weight was monitored over a period of 7 months (n=10 mice per group, *P<0.05 compared to control group, two-way repeated-measures ANOVA with Bonferroni post-tests, the means±s.d. are depicted). (c) Peripheral blood was collected from control and BDE-209-exposed mice at month 7, and the hematological parameters, including leukocytes (WBC), red blood cells (RBC), platelets (PLT), hemoglobin (HGB), packed cell volume (PCV) and mean corpuscular volume (MCV), were detected by a CAD (**P<0.01, unpaired Student's t-test, the means±s.d. are depicted). BDE, brominated diphenyl ether; bw, body weight; CAD, Coulter AcT Diff hematology analyzer; GC–MS, gas chromatography-mass spectrometry; NCI, negative chemical ionization.
To determine whether continuous exposure to BDE-209 and its accumulation in the plasma have any adverse effects on the hematopoietic system, hematological parameters were examined at month 7. There were no significant differences in the number of red blood cells (RBCs), platelets (PLTs), hemoglobin (HGB), the packed cell volume (PCV) or the mean corpuscular volume (MCV) between BED-209-exposed mice and the controls (Figure 1c). However, BDE-209-exposed mice had significant lower numbers of leukocytes (also known as white blood cells) compared to the control mice (8.21±2.51×109/l versus 12.71±2.69×109/l, P<0.01).
Therefore, these data indicate that continuous exposure to BDE-209 results in the accumulation of high levels of BDE-209 in the plasma, reduced body weight and fewer numbers of leukocytes in the peripheral blood, but it has only minimal effects on other components of the hematopoietic system.
BDE-209 exposure specifically affects peripheral immune cells
To further investigate the effect of BDE-209 exposure on leukocytes, we performed a dose–response study by exposing mice to a dose of 8, 80 or 800 mg/kg bw of BDE-209 at 2-day intervals and examined the immune cells in the peripheral blood, spleen and thymus. Compared to the controls (0 mg/kg bw), significantly lower numbers of monocytes were observed in the peripheral blood of experimental mice after receiving doses of 8, 80 or 800 mg/kg bw for 1 or 2 months. However, there was no significant difference in the number of lymphocytes, neutrophils and eosinophils between BDE-209-exposed mice and controls at these time points (Figure 2b and data not shown). At month 3, the mice that were exposed to BDE-209 at doses of 80 or 800 mg/kg bw had significantly lower numbers of CD4 T, CD8 T and B lymphocytes in the spleen compared to the controls (Figure 2c). Additionally, lower percentages and numbers of CD11b+Ly6Chi monocytes, CD11b+Ly6Cmid neutrophils and CD11b+CD11c+ dendritic cells were observed in the spleens of mice exposed to BDE-209 at doses of 80 or 800 mg/kg bw compared to the controls (Figure 2d and data not shown).
The effects of BDE-209 exposure on the body weight and the number of immune cells in the peripheral blood and spleen. BDE-209-exposed mice were intragastrically administered BDE-209 at doses of 8, 80 or 800 mg/kg bw at 2-day intervals. (a) The body weight was evaluated at month 3. (b) The immune cells, including lymphocytes, neutrophils, monocytes and eosinophils, in the peripheral blood were detected by a HEMAVET 950 multispecies hematology instrument (DREW Scientific Inc.) at month 1. (c, d) Splenocytes were collected from control mice and BDE-209-exposed mice at month 3, and the numbers of CD4 T (CD3+CD4+), CD8 T (CD3+CD8+) and B cells (B220+), as well as monocytes (CD11b+Ly6Chi), neutrophils (CD11b+Ly6Cmid) and dendritic cells (CD11b+CD11c+) in the spleen were calculated by flow cytometry through staining with anti-CD3e, anti-CD4, anti-CD8a, anti-B220, anti-CD11b, anti-Ly6C and anti-CD11c (*P<0.05, **P<0.01, one-way ANOVA followed by Tukey's post-tests, the means±s.d. are depicted). BDE, brominated diphenyl ether; bw, body weight.
No body weight differences were observed between the controls and the mice that were exposed to different doses of BDE-209 for the first 3 months of the study (Figure 2a). Furthermore, there were no significant differences in thymocytes; the percentages and absolute numbers of cells of T-cell subpopulations (CD4+ single-positive, CD8+ single-positive and CD4+CD8+ double-positive), CD11b+Ly6G+ neutrophils and CD11b+CD11c+ dendritic cells in the thymus were similar between the control and BDE-209-exposed mice (Supplementary Figures 2 and 3). Together, these results indicate that BDE-209 exposure specifically affects peripheral immune cells and that this effect is not due to overt toxicity.
Long-term exposure to BDE-209 has adverse effects on the functionality of CD8 T cells
CD8 T lymphocytes play a pivotal role in the host immune defense against infection and tumor. Flow cytometry was used to examine the effects of BDE-209 exposure on the functionality of CD8 T cells. Our results showed that after 7 months of BDE-209 exposure, the CD8 T cells in the peripheral blood from mice exposed to BDE-209 at 800 mg/kg bw produced less of the cytokines IFN-γ and IL-2 (Figure 3a) than CD8 T cells from the controls, as determined by intracellular cytokine staining after in vitro stimulation with PMA and ionomycin. In addition, at the endpoint of the 27-month exposure, the splenic CD8 T cells from these experimental mice produced much less IFN-γ and IL-2 than the controls (Supplementary Figure 4).
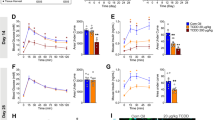
Long-term exposure to BDE-209 at a dose of 800 mg/kg bw had adverse effects on the functionality of CD8 T cells in the peripheral blood. The cytokine production by CD8 T cells in the peripheral blood was measured by ICS following stimulation with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 5 h in vitro. (a) Comparison of the IFN-γ and IL-2 expression by CD8+ T cells between the control mice and the BDE-209-exposed mice at month 7. As shown in the flow diagram (gated on CD8+ T cells), the percentages (%) indicate the IFN-γ- or IL-2-positive population of CD8+ T cells (left panels). The bar graphs display the corresponding statistical results of IFN-γ+ CD8+ T cells or IL-2+ CD8+ T cells between the control and BDE-209-exposed mice. (b) Polyfunctional CD8 T cells were detected by ICS with multicolor flow cytometry in control and BDE-209-exposed mice at month 7. The percentages of four combinations of IFN-γ, IL-2 and TNF-α expression of CD8+ T cells are shown in the pie charts (three cytokines: IFN-γ+TNF-α+IL-2+; two cytokines: IFN-γ+TNF-α+IL-2−, IFN-γ+TNF-α−IL-2+ and IFN-γ−TNF-α+IL-2+; one cytokine: IFN-γ+TNF-α−IL-2−, IFN-γ−TNF-α−IL-2+ and IFN-γ−TNF-α+IL-2−; no cytokines: IFN-γ−TNF-α−IL-2−). (c) Kinetics of the percentages of IFN-γ+CD8+ (left) or IL-2+CD8+ (right) T cells from months 1 to 5 in the control and BDE-209-exposed mice. The data in a were analyzed using an unpaired Student's t-test, and the data in c were assessed using two-way repeated-measures ANOVA (with Bonferroni post-tests) (n=10 per group, the means±s.d. are depicted, *P<0.05 and **P<0.01 compared to the control group). BDE, brominated diphenyl ether; bw, body weight; ICS, intracellular cytokine staining.
CD8 T cells are capable of producing multiple cytokines upon T-cell receptor stimulation and play important roles in controlling infection by intracellular pathogens and eliminating cancerous cells. Recent studies show that polyfunctional T cells capable of expressing multiple cytokines provide better protection against infectious diseases and tumors,27,28,29 while certain conditions, such as continued stimulation during chronic infection or long-term tumor progression, can reduce the ability of CD8 T cells to produce multiple cytokines.30,31 Compared to the controls, we observed a significant decrease in the number of CD8 T cells that simultaneously produced three (IFN-γ+TNF-α+IL-2+) or two (IFN-γ+TNF-α+IL-2−, IFN-γ+TNF-α−IL-2+ or IFN-γ−TNF-α+IL-2+) cytokines in the peripheral blood from these mice after 7 months of exposure to BDE-209 at a dose of 800 mg/kg bw every other day (Figure 3b).
To determine whether the effect on CD8 T-cell functions is due to immediate suppression by the presence of BDE-209 in the plasma or to long-term BDE-209 exposure, we analyzed the cytokine production of CD8 T cells in the peripheral blood from mice that had been exposed to BDE-209 for 1–5 months. There were no significant differences in cytokine production between the controls and BDE-209 exposed mice at the early time points of 1 and 2 months when the accumulation of BDE-209 in the plasma had already reached the high, steady-state level. Significant differences in IFN-γ and IL-2 production were observed only after more than 4 months of BDE-209 exposure (Figure 3c). These results indicated that long-term BDE-209 exposure decreased the polyfunctional CD8 T-cell population in adult mice.
Continuous BDE-209 exposure reduces the proliferative capacity of CD8 T cells
In addition to cytokine production, another important feature of T cells is proliferation upon stimulation to produce a large numbers of effector cells in response to foreign insults. We tested the effect of continuous BDE-209 exposure on the proliferative capacity of CD8 T cells upon stimulation. At month 9, PBMCs from control mice and mice exposed to BDE-209 at 800 mg/kg bw were labeled with CFSE and stimulated in vitro with anti-CD3 and anti-CD28 for 0, 48 or 72 h. The proliferation of CD8 T cells was visualized by FACS analysis of CFSE fluorescence in CD8+ cells (Figure 4a). A large population of CD8 T cells from the control and BDE-209-exposed mice divided after 48 h of stimulation, as indicated by a loss of CFSE fluorescence. By 72 h, almost all of the CD8 T cells from the control mice had divided. In contrast, a small population of CD8 T cells from the BDE-209-exposed mice had high levels of CFSE fluorescence at 48 and 72 h, indicating that they had failed to proliferate in response to stimulation (Figure 4a).
Continuous BDE-209 exposure impaired proliferation and induced apoptosis of CD8 T cells. (a) PBMCs from control mice and mice administered BDE-209 at a dose of 800 mg/kg bw were labeled with CFSE (5 µM) and stimulated with pre-bound anti-CD3e (2 µg/ml) plus soluble anti-CD28 (5 µg/ml) for 0, 48 or 72 h. The proliferation of CD8 T cells was visualized by FACS analysis of CFSE fluorescence in CD8+ cells (solid lines, control mice; dotted lines, BDE-209-exposed mice). (b, c) Mice were intragastrically administered BDE-209 at a dose of 8, 80 or 800 mg/kg bw at 2-day intervals, and splenic CD8 T cells were purified from the control mice and BDE-209-exposed mice using a FACS Aria II based on the surface expression of CD3 and CD8 (CD3+CD8+) at month 3. The purified splenic-CD8 T cells were labeled with CFSE (5 µM) and then stimulated with anti-CD3 and anti-CD28 mAbs coupled to magnetic beads at a cell-bead ratio of 4∶1 for 72 h (dotted lines, unstimulated splenic CD8+ T cells). The proliferation (b) and the proportions of early (Annexin V+PI−) and late apoptotic or necrotic (Annexin V+PI+) cells (c) were evaluated by FACS analysis of the CD8+ cells. Each group included five mice; representative images are shown. BDE, brominated diphenyl ether; bw, body weight; PBMC, peripheral blood mononuclear cell.
The reduced proliferation of CD8 T cells from the BDE-209-exposed mice could be due to an intrinsic defect of the CD8 T cells or to alterations of other cell types that provide second (costimulatory) and third (cytokine) signals in addition to the T-cell receptor signal provided by anti-CD3 and anti-CD28 mAb in the in vitro stimulation assay. To distinguish these two possibilities, splenic CD8 T cells were purified by FACS sorting, labeled with CFSE, and stimulated in vitro with anti-CD3 and anti-CD28 for 72 h. CD8 T cells from mice exposed to high doses (80 and 800 mg/kg bw) of BDE-209 for 3 months had reduced level of proliferation; 50%–60% of the CD8 T cells had divided compared to 70% of the CD8 T cells from the controls (Figure 4b). Furthermore, only 10%–15% of the CD8 T cells from BDE-209-exposed mice (80 and 800 mg/kg bw) divided more than once, compared to 33% of the cells from the control mice. Reduced CD8 T cell proliferation is accompanied by increased apoptosis, and higher proportions of early (Annexin V+PI−) and late apoptotic or necrotic (Annexin V+PI+) cells were observed in CD8 T cells from BDE-209-exposed mice at month 3 (Figure 4c). These data indicate that BDE-209 exposure impairs the proliferative capacity of CD8 T cells and renders them more prone to apoptosis upon stimulation.
Long-term exposure to BDE-209 decreases memory phenotype CD8 T cells
The peripheral T cell population consists of T cells with naive (CD44lo) and memory (CD44hi) phenotypes. Memory (CD44hi) T cells are further divided into distinct populations of central memory (TCM) and effector memory (TEM) cells that express high and low levels of CD62L, respectively. TCM and TEM have different effector functions and homing capacities.32,33 TEM cells are first responders that rapidly produce high levels of effector cytokines, while TCM cells have a high proliferative capacity and, thus, produce more secondary effector cells that reinforce the host response to foreign insults. We examined the effect of BDE-209 exposure on TCM and TEM cells, and the results showed that the numbers of both central memory (TCM, CD44hiCD62Lhi) and effector memory (TEM, CD44hiCD62Llo) CD8 T cells from the peripheral blood were less in mice exposed to BDE-209 at a dose of 800 mg/kg bw than in control mice at month 7 (Figure 5). Similar results were observed when the TCM and TEM subsets were examined based on CCR7 and CD27 expression; TCM cells express high levels of CCR7 and CD27, and TEM cells express low levels of CCR7 and CD27 (Supplementary Figure 5a and b).34,35 The homeostasis and maintenance of memory-phenotype CD8 T cells is dependent on IL-2, IL-15 and IL-7. The expression of the receptors for these cytokines, CD122 (IL-2Rβ/IL-15R) and CD127 (IL-7Rα), on CD8 T cells was slightly lower in BDE-209-exposed mice compared to the controls (Supplementary Figure 5c). These data indicate that long-term BDE-209 exposure decreases both the TCM and TEM phenotypes of CD8 T cells, possibly by affecting their ability to undergo homeostasis.
Long-term BDE-209 exposure at a dose of 800 mg/kg bw decreased the abundance of memory-phenotype CD8 T cells in the peripheral blood. The percentages of central memory (TCM, CD44hiCD62Lhi) and effector memory (TEM, CD44hiCD62Llo) phenotype CD8 T cells were determined by flow cytometry. (a) Flow diagrams (left) and bar graphs (right) show the percentages of TCM and TEM CD8 T cells in control and BDE-209-exposed mice at month 7 (n=8 per group, *P<0.05, unpaired Student's t-test, the means±s.d.). (b) Kinetics of the percentages of TCM and TEM in CD8 T cells from months 1 to 7 in control and BDE-209-exposed mice (n=8 per group, *P<0.05 compared to the control group, two-way repeated-measures ANOVA with Bonferroni post-tests, the means±s.d. are depicted). BDE, brominated diphenyl ether; bw, body weight.
Long-term BDE-209 exposure impairs antigen-specific CD8 T-cell responses
To determine whether the generation of antigen-specific CD8 T cells was also affected by long-term BDE-209 exposure, we infected control and BDE-209-exposed mice with rLm-OVA at month 10. OVA257–264-specific CD8 T cells were detected by MHC/peptide tetramers or intracellular cytokine staining at day 7 after infection. Mice exposed to BDE-209 at a dose of 800 mg/kg bw had a lower frequency of OVA257–264-specific CD8 T cells compared to the control mice as detected by Kb/OVA257–264 tetramer staining (Figure 6a), indicating a weaker antigen-specific CD8 T-cell response in BDE-209-exposed mice. Furthermore, OVA257–264-specific CD8 T cells from mice exposed to BDE-209 were less functional compared to those from control mice. They produce less IFN-γ as indicated by intracellular IFN-γ (IFN-γ+/OVA257–264) staining (Figure 6b), and significantly fewer OVA257–264-specific CD8 T cells produce both IFN-γ and TNF-α in BDE-209-exposed mice (Figure 6c). These data showed that mice with long-term BDE-209-exposure mounted a weaker and less functional antigen-specific CD8 T-cell response to infection.
Long-term BDE-209 exposure impairs antigen-specific CD8 T-cell responses. At month 10, control mice and mice exposed to BDE-209 at a dose of 800 mg/kg bw were infected with rLm-OVA (intravenously) at a dose of 5×104 CFU/mouse. Seven days later, OVA257–264-specific CD8 T cells in peripheral blood were detected by MHC/peptide tetramers or intracellular cytokine staining. (a, b) Detection of OVA257–264-specific CD8 T cells by MHC/peptide tetramers (Kb/OVA257–264, a) or intracellular IFN-γ (IFN-γ+/OVA257–264, b) staining. (c) Detection of OVA257–264-specific polyfunctional CD8 T cells by intracellular cytokine staining with multicolor flow cytometry. Polyfunctional CD8 T cells were considered the population of IFN-γ+TNF-α+ (double-positive) cells gated on CD8+ T cells. The data were assessed using an unpaired Student's t-test (n=8 per group, the means±s.d. are depicted, *P<0.05, **P<0.01). BDE, brominated diphenyl ether; bw, body weight.
Discussion
Our results showed that continuous exposure of adult mice to BDE-209 led to the accumulation of BDE-209 that was maintained at a high, steady-state level in the blood. In the general human population, the concentration of BDE-209 in the serum is low, e.g., a median of 5.0 ng/g lipid in Norway, 9.94 ng/g lipid in Sweden and 0.90 ng/g lipid in Japan.5,36 However, the level of BDE-209 is extremely high in certain populations that are subject to occupational or regional industry exposure. For example, the BDE-209 level in residents of Guiyu, an electronic waste dismantling region in South China, is as high as 3100 ng/g lipid in the serum,11 which is 3444 times higher than the 0.90 ng/g lipid found in the sera of the Japanese general population. Our model of continuous exposure by intragastric administration with a dose of 800 mg/kg bw at 2-day intervals resulted in a steady-state level of approximately 200–300 ng/ml or 40 000–60 000 ng/g lipid, which is approximately 123–184 times higher than the median that was found in catering workers from Southeast China (1.63 ng/ml or 307 ng/g lipid).37 These comparisons based on the available data indicate that the burden of BDE-209 in the plasma of our model is higher than what is found in the general human population, but is approaching the level that is found in people who work in professions with high exposure.
Our results show that continuous BDE-209 exposure and accumulation in the blood results in reduced leukocytes. A recent study showed that the numbers of monocytes in the peripheral blood were decreased, although the thymic T cell populations (CD4+CD8−, CD4−CD8+, CD4+CD8+ and CD4−CD8−) were not altered, after oral gavage with DE-71 (a commercial penta-BDE mixture) at doses of 0.018, 0.18 or 1.8 mg/kg/day for 28 day in B(6)C(3)F(1) mice.23 An earlier study also found that exposure to 2,2′,4,4′-tetrabromodiphenyl ether at a dose of 36 mg/kg bw/day for 14 days reduced the numbers of splenocytes and CD4 T, CD8 T and B cells in the spleen, but it did not affect the number of thymocytes or the T cell subpopulations in the thymus of adult C57BL/6 mice.38 Additionally, Bondy et al.39 reported that perinatal exposure to the brominated diphenyl ether (BDE) mixture DE-71 at a dose of 5 or 25 mg/kg bw/day decreased the number of splenic B cells in Sprague–Dawley rats. A longitudinal cohort study of 33 children has also shown that serum ΣPBDEs levels were negatively associated with the lymphocyte count.21 These results are consistent with our findings that continuous BDE-209 exposure reduced the number of leukocytes in peripheral blood, the numbers of CD4 T, CD8 T and B lymphocytes or CD11b+Ly6Chi monocytes, CD11b+Ly6Cmid neutrophils and CD11b+CD11c+ dendritic cells in the spleen, but did not affect the numbers of thymocytes and T-cell subpopulations (CD4 SP, CD8 SP and CD4CD8 DP) in the thymus of adult C57BL/6 female mice.
In our further studies on the effect of BDE-209 exposure on leukocytes, we found that continuous BDE-209 exposure impaired the functionality and reduced the proliferative capacity of CD8 T cells. Suppressed T lymphocyte multiplication was also observed in rats during pregnancy and lactation following exposure to BDE-209 at a dose of 300 mg/kg bw/day in vivo.20 Moreover, although Fair et al.23 reported an increase in mitogen-stimulated T cell proliferation in mice after short-term exposure to DE-71 by oral gavage at low doses of 1.8 or 3.6 mg/kg bw/day for 28 days, no effect on the mitogen-induced proliferation of human lymphocytes was observed in another study after in vitro exposure to individual PBDEs.40 The variation between the results observed in these studies is likely due to the different models and parameters used, particularly the durations and doses of PBDE exposure. Our results showed that continuous BDE-209 exposure at doses of 80 or 800 mg/kg bw at 2-day intervals reduced the proliferation of CD8 T cells. Furthermore, impaired functionality and a reduced number of memory phenotype CD8 T cells were observed after long-term BDE-209 exposure. These results raise the concern of additional risks for populations with sustained exposure to high levels of BDE-209 due to related occupational or regional industries and call for further studies using a long-term or subchronic continuous exposure model instead of the short-term acute exposure that is commonly used in current studies.
The reduced proliferation and functionality of CD8 T cells observed in this study suggest that BDE-209 exposure may affect the ability of the host to mount an effective, antigen-specific CD8 T cell response to infection. Indeed, our results show that mice with long-term BDE-209 exposure mount weaker and less functional antigen-specific CD8 T-cell responses to infections. Thus, both the quantity and quality of the antigen-specific CD8 T-cell response were reduced by long-term BDE-209 exposure. Consistent with our findings, previous studies have also suggested PBDE exposure has a negative impact on the host defense against infection. Berg et al.41 observed a high prevalence of infections and pathological changes in burbot (Lotalota) from Lake Mjøsa, which is contaminated by a high level of PBDEs, compared to burbot from the less polluted Lake Losna. Lundgren et al.42 found that the adult mice exposed to a high dose of BDE-99 had higher virus levels in liver than those exposed to a low dose after infection with coxsackievirus B3.
Taken together, we found that mice continuously exposed to BDE-209 sustained a higher burden of BDE-209 in the plasma and had reduced leukocytes, lower cytokine (IFN-γ, IL-2 and TNF-α) production, weaker CD8 T-cell proliferation, decreased memory phenotype CD8 T cells and impaired antigen-specific CD8 T-cell responses compared to control animals. These results show that continuous BDE-209 exposure has adverse effects on the immune system and may weaken the ability of the host to combat infections and tumors. Thus, the immunological status and the infection and tumor rates of the occupational workers and residents with high exposure to PBDEs should be carefully evaluated. Further studies are needed to investigate the mechanisms by which BDE-209 exposure harms the immune cells and to explore means to prevent and/or reverse the immune suppression.
References
Rahman F, Langford KH, Scrimshaw MD, Lester JN . Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ 2001; 275: 1–17.
Costa LG, Giordano G . Is decabromodiphenyl ether (BDE-209) a developmental neurotoxicant? Neurotoxicology 2011; 32: 9–24.
Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E et al. High body burdens of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in California women. Environ Health Perspect 2003; 111: 1175–1179.
Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE et al. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect 2004; 112: 654–658.
Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE . Human internal and external exposure to PBDEs—a review of levels and sources. Int J Hyg Environ Health 2009; 212: 109–134.
Devanathan G, Subramanian A, Sudaryanto A, Takahashi S, Isobe T, Tanabe S . Brominated flame retardants and polychlorinated biphenyls in human breast milk from several locations in India: potential contaminant sources in a municipal dumping site. Environ Int 2012; 39: 87–95.
Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J . Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med 2005; 47: 199–211.
Wang J, Ma YJ, Chen SJ, Tian M, Luo XJ, Mai BX . Brominated flame retardants in house dust from e-waste recycling and urban areas in South China: implications on human exposure. Environ Int 2010; 36: 535–541.
Bi X, Thomas GO, Jones KC, Qu W, Sheng G, Martin FL et al. Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ Sci Technol 2007; 41: 5647–5653.
Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A . Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed 2008; 79: 172–183.
Costa LG, Giordano G . Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 2007; 28: 1047–1067.
Chen Q, Yu L, Yang L, Zhou B . Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat Toxicol 2012; 110–111: 141–148.
Wu JP, Luo XJ, Zhang Y, Yu M, Chen SJ, Mai BX et al. Biomagnification of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls in a highly contaminated freshwater food web from South China. Environ Pollut 2009; 157: 904–909.
Zhang W, Cai Y, Sheng G, Chen D, Fu J . Tissue distribution of decabrominated diphenyl ether (BDE-209) and its metabolites in sucking rat pups after prenatal and/or postnatal exposure. Toxicology 2011; 283: 49–54.
He J, Yang D, Wang C, Liu W, Liao J, Xu T et al. Chronic zebrafish low dose decabrominated diphenyl ether (BDE-209) exposure affected parental gonad development and locomotion in F1 offspring. Ecotoxicology 2011; 20: 1813–1822.
Stapleton HM, Letcher RJ, Li J, Baker JE . Dietary accumulation and metabolism of polybrominated diphenyl ethers by juvenile carp (Cyprinus carpio). Environ Toxicol Chem 2004; 23: 1939–1946.
Chen A, Dietrich KN, Huo X, Ho SM . Developmental neurotoxicants in e-waste: an emerging health concern. Environ Health Perspect 2011; 119: 431–438.
Eng ML, Elliott JE, MacDougall-Shackleton SA, Letcher RJ, Williams TD . Early exposure to 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) affects mating behavior of zebra finches. Toxicol Sci 2012; 127: 269–276.
Ernest SR, Wade MG, Lalancette C, Ma YQ, Berger RG, Robaire B et al. Effects of chronic exposure to an environmentally relevant mixture of brominated flame retardants on the reproductive and thyroid system in adult male rats. Toxicol Sci 2012; 127: 496–507.
Liu X, Zhan H, Zeng X, Zhang C, Chen D . The PBDE-209 exposure during pregnancy and lactation impairs immune function in rats. Mediators Inflamm 2012; 2012: 692467.
Leijs MM, Koppe JG, Olie K, van Aalderen WM, de Voogt P, ten Tusscher GW . Effects of dioxins, PCBs, and PBDEs on immunology and hematology in adolescents. Environ Sci Technol 2009; 43: 7946–7951.
van der Ven LT, van de Kuil T, Leonards PE, Slob W, Canton RF, Germer S et al. A 28-day oral dose toxicity study in Wistar rats enhanced to detect endocrine effects of decabromodiphenyl ether (decaBDE). Toxicol Lett 2008; 179: 6–14.
Fair PA, Stavros HC, Mollenhauer MA, DeWitt JC, Henry N, Kannan K et al. Immune function in female B(6)C(3)F(1) mice is modulated by DE-71, a commercial polybrominated diphenyl ether mixture. J Immunotoxicol 2012; 9: 96–107.
Pearce EL, Shen H . Generation of CD8 T cell memory is regulated by IL-12. J Immunol 2007; 179: 2074–2081.
Yu Y, Han S, Zhang D, van de Wiele T, Lu M, Wang D et al. Factors affecting the bioaccessibility of polybrominated diphenylethers in an in vitro digestion model. J Agric Food Chem 2009; 57: 133–139.
Yu YX, Huang NB, Zhang XY, Li JL, Yu ZQ, Han SY et al. Polybrominated diphenyl ethers in food and associated human daily intake assessment considering bioaccessibility measured by simulated gastrointestinal digestion. Chemosphere 2011; 83: 152–160.
Almeida JR, Sauce D, Price DA, Papagno L, Shin SY, Moris A et al. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood 2009; 113: 6351–6360.
Betts MR, Nason MC, West SM, de Rosa SC, Migueles SA, Abraham J et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006; 107: 4781–4789.
Darrah PA, Patel DT, de Luca PM, Lindsay RW, Davey DF, Flynn BJ et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13: 843–850.
Zhang Y, Huang S, Gong D, Qin Y, Shen Q . Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol 2010; 7: 389–395.
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439: 682–687.
DiSpirito JR, Shen H . Quick to remember, slow to forget: rapid recall responses of memory CD8+ T cells. Cell Res 2010; 20: 13–23.
Sallusto F, Geginat J, Lanzavecchia A . Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22: 745–763.
Kaech SM, Wherry EJ, Ahmed R . Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol 2002; 2: 251–262.
Northrop JK, Shen H . CD8+ T-cell memory: only the good ones last. Curr Opin Immunol 2004; 16: 451–455.
Uemura H, Arisawa K, Hiyoshi M, Dakeshita S, Kitayama A, Takami H et al. Congener-specific body burden levels and possible determinants of polybrominated diphenyl ethers in the general Japanese population. Chemosphere 2010; 79: 706–712.
Wang C, Lin Z, Dong Q, Lin K, Wang J, Huang J et al. Polybrominated diphenyl ethers (PBDEs) in human serum from Southeast China. Ecotoxicol Environ Saf 2012; 78: 206–211.
Thuvander A, Darnerud PO . Effects of polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) on some immunological parameters after oral exposure in rats and mice. Toxicol Environ Chem 1999; 70: 229–242.
Bondy GS, Lefebvre DE, Aziz S, Cherry W, Coady L, Maclellan E et al. Toxicologic and immunologic effects of perinatal exposure to the brominated diphenyl ether (BDE) mixture DE-71 in the Sprague–Dawley rat. Environ Toxicol 2013; 28: 215–228.
Fernlof G, Gadhasson I, Podra K, Darnerud PO, Thuvander A . Lack of effects of some individual polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) congeners on human lymphocyte functions in vitro. Toxicol Lett 1997; 90: 189–197.
Berg V, Zerihun MA, Jorgensen A, Lie E, Dale OB, Skaare JU et al. High prevalence of infections and pathological changes in burbot (Lota lota) from a polluted lake (Lake Mjosa, Norway). Chemosphere 2013; 90: 1711–1718.
Lundgren M, Darnerud PO, Ilback NG . The flame-retardant BDE-99 dose-dependently affects viral replication in CVB3-infected mice. Chemosphere 2013; 91: 1434–1438.
Acknowledgements
We thank Yingxin Yu (Institute of Environmental Pollution and Health, School of Environmental and Chemical Engineering, Shanghai University) for determining the BDE-209 concentration, Nining Guo and Qibing Leng (Institute Pasteur of Shanghai, Chinese Academy of Science) for the rLm-OVA infections and Xing Zhang (College of Public Health, Shanghai Jiaotong University School of Medicine) for breeding the animals. This work is supported by the National Basic Research Program of China (2011CB512104), the 12th Five-Year Plan of National Science and Technology Support (2012BAK17B10) and the National Natural Science Foundation of China (81101252).
Author information
Authors and Affiliations
Additional information
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, user swill need toobtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0
About this article
Cite this article
Zeng, W., Wang, Y., Liu, Z. et al. Long-term exposure to decabrominated diphenyl ether impairs CD8 T-cell function in adult mice. Cell Mol Immunol 11, 367–376 (2014). https://doi.org/10.1038/cmi.2014.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2014.16
Keywords
This article is cited by
-
Persistent immune injury induced by short-term decabromodiphenyl ether (BDE-209) exposure to female middle-aged Balb/c mice
Environmental Science and Pollution Research (2023)
-
PDL1 blockage increases fetal resorption and Tfr cells but does not affect Tfh/Tfr ratio and B-cell maturation during allogeneic pregnancy
Cell Death & Disease (2020)
-
Physiological function of phospholipase D2 in anti-tumor immunity: regulation of CD8+ T lymphocyte proliferation
Scientific Reports (2018)
-
Peripheral CD19hi B cells exhibit activated phenotype and functionality in promoting IgG and IgM production in human autoimmune diseases
Scientific Reports (2017)
-
Characterization of T follicular helper cells in allogeneic normal pregnancy and PDL1 blockage-induced abortion
Scientific Reports (2016)