Abstract
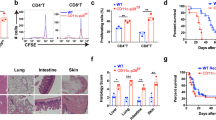
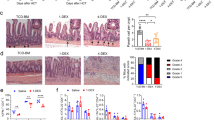
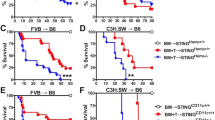
Graft-versus-host disease (GVHD) is the most common complication after hematopoietic stem cell transplantation. To clarify the role of Toll-like receptor 4 (TLR4), which is a major receptor for bacterial lipopolysaccharides (LPS), in the development of acute GVHD, we used a TLR4-knockout (TLR4−/−) mouse GVHD model and analyzed the underlying immunological mechanisms. When TLR4−/− mice were used as bone marrow and splenocyte cell graft donors or recipients, GVHD symptom occurrence and mortality were delayed compared to wild-type (TLR4+/+) mice. In addition, histopathological analyses revealed that in TLR4−/−→BALB/c chimeras, liver and small intestine tissue damage was reduced with minimal lymphocytic infiltration. In contrast to TLR4+/+, TLR4−/− mice dendritic cells did not express CD80, CD86, CD40, MHC-II or IL-12 during LPS induction and remained in an immature state. Furthermore, the ability of TLR4−/− mice spleen dendritic cells to promote allogeneic T-cell proliferation and, in particular, T-helper cell 1 (Th1) development was obviously attenuated compared with TLR4+/+ mice dendritic cells, and the levels of interferon-γ (IFN-γ) and IL-10, Th2-cell specific cytokines, were significantly higher in the serum of TLR4−/−→BALB/c than in TLR4+/+→BALB/c chimeric mice. Overall, our data revealed that TLR4 may play a role in the pathogenesis of GVHD and that targeted TLR4 gene therapy might provide a new treatment approach to reduce the risk of GVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Paczesny S, Hanauer D, Sun Y, Reddy P . New perspectives on the biology of acute GVHD. Bone Marrow Transplant 2010; 45: 1–11.
Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS . Leukocyte migration and graft-versus-host disease. Blood 2005; 105: 4191–4199.
Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood 2005; 106: 1113–1122.
Auffermann-Gretzinger S, Lossos IS, Vayntrub TA, Leong W, Grumet FC, Blume KG et al. Rapid establishment of dendritic cell chimerism in allogeneic hematopoietic cell transplant recipients. Blood 2002; 99: 1442–1448.
Gonzalez B, Guerra C, Morris D . Dendritic cells in infectious disease, hypersensitivity, and autoimmunity. Int J Interferon Cytokine Mediator Res 2010; 2: 137–147.
Mullighan CG, Bardy PG . New directions in the genomics of allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2007; 13: 127–144.
Lu YC, Yeh WC, Ohashi PS . LPS/TLR4 signal transduction pathway. Cytokine 2008; 42: 145–151.
Iwasaki A, Medzhitov R . Regulation of adaptive immunity by the innate immune system. Science 2010; 327: 291–295.
Ferrara JL, Reddy P . Pathophysiology of graft-versus-host disease. Semin Hematol 2006; 43: 3–10.
Cooke KR, Olkiewicz K, Erickson N, Ferrara JL . The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res 2002; Sect. 441–448.
Netea MG, van Deuren M, Kullberg BJ, Cavaillon JM, van der Meer JW . Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol 2002; 23: 135–139.
Beutler B, Poltorak A . The sole gateway to endotoxin response: how LPS was identified as Tlr4, and its role in innate immunity. Drug Metab Dispos 2001; 29: 474–478.
Ferwerda B, McCall MB, Verheijen K, Kullberg BJ, van der Ven A, van der Meer JW et al. Functional consequences of Toll-like receptor 4 polymorphisms. Mol Med 2008; 14: 346–352.
Raby BA, Klimecki WT, Laprise C, Renaud Y, Faith J, Lemire M et al. Polymorphisms in Toll-like receptor 4 are not associated with asthma or atopy-related phenotypes. Am J Respir Crit Care Med 2002; 166: 1449–1456.
Carvalho A, Marques A, Maciel P, Rodrigues F . Study of disease-relevant polymorphisms in the TLR4 and TLR9 genes: a novel method applied to the analysis of the Portuguese population. Mol Cell Probes 2007; 21: 316–320.
Berdeli A, Emingil G, Han Saygan B, Gurkan A, Atilla G, Kose T et al. TLR2 Arg753Gly, TLR4 Asp299Gly and Thr399Ile gene polymorphisms are not associated with chronic periodontitis in a Turkish population. J Clin Periodontol 2007; 34: 551–557.
Nebel A, Flachsbart F, Schafer A, Nothnagel M, Nikolaus S, Mokhtari NE et al. Role of the toll-like receptor 4 polymorphism Asp299Gly in longevity and myocardial infarction in German men. Mech Ageing Dev 2007; 128: 409–411.
Awomoyi AA, Rallabhandi P, Pollin TI, Lorenz E, Sztein MB, Boukhvalova MS et al. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol 2007; 179: 3171–3177.
Rezazadeh M, Hajilooi M, Rafiei A, Haidari M, Nikoopour E, Kerammat F et al. TLR4 polymorphism in Iranian patients with brucellosis. J Infect 2006; 53: 206–210.
Okayama N, Fujimura K, Suehiro Y, Hamanaka Y, Fujiwara M, Matsubara T et al. Simple genotype analysis of the Asp299Gly polymorphism of the Toll-like receptor-4 gene that is associated with lipopolysaccharide hyporesponsiveness. J Clin Lab Anal 2002; 16: 56–58.
Kim YS, Hwang YJ, Kim SY, Yang SM, Lee KY, Park Ie B . Rarity of TLR4 Asp299Gly and Thr399Ile polymorphisms in the Korean population. Yonsei Med J 2008; 49: 58–62.
Elmaagacli AH, Koldehoff M, Hindahl H, Steckel NK, Trenschel R, Peceny R et al. Mutations in innate immune system NOD2/CARD 15 and TLR-4 (Thr399Ile) genes influence the risk for severe acute graft-versus-host disease in patients who underwent an allogeneic transplantation. Transplantation 2006; 81: 247–254.
Imado T, Iwasaki T, Kitano S, Satake A, Kuroiwa T, Tsunemi S et al. The protective role of host Toll-like receptor-4 in acute graft-versus-host disease. Transplantation 2010; 90: 1063–1070.
Lorenz E, Schwartz DA, Martin PJ, Gooley T, Lin MT, Chien JW et al. Association of TLR4 mutations and the risk for acute GVHD after HLA-matched-sibling hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001; 7: 384–387.
Calcaterra C, Sfondrini L, Rossini A, Sommariva M, Rumio C, Menard S et al. Critical role of TLR9 in acute graft-versus-host disease. J Immunol 2008; 181: 6132–6139.
Yaa MN, Paolo P, Peter S, Eric PG, Jaya S, Papanicolaou GA . Toll-like receptor 4 polymorphisms and risk of Gram-negative bacteremia after allogeneic stem cell transplantation. A prospective pilot study. Biol Blood Marrow Transplant 2009; 15: 1130–1133.
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 1999; 162: 3749–3752.
Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J Jr, Crawford JM et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood 1996; 88: 3230–3239.
Asai O, Longo DL, Tian ZG, Hornung RL, Taub DD, Ruscetti FW et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest 1998; 101: 1835–1842.
Bryson JS, Jennings CD, Lowery DM, Carlson SL, Pflugh DL, Caywood BE et al. Rejection of an MHC class II negative tumor following induction of murine syngeneic graft-versus-host disease. Bone Marrow Transplant 1999; 23: 363–372.
Xu S, Liu X, Bao Y, Zhu X, Han C, Zhang P et al. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps–SHP-2 pathway. Nat Immunol 2012; 13: 551–559.
Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat Immunol 2011; 12: 416–424.
Han C, Jin J, Xu S, Liu H, Li N, Cao X . Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol 2010; 11: 734–742.
Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol 2011; 12: 861–869.
Zhang M, Tang H, Guo Z, An H, Zhu X, Song W et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol 2004; 5: 1124–1133.
Hamza T, Barnett JB, Li B . Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol Sci 2010; 11: 789–806.
Austrup F, Vestweber D, Borges E, Lhning M, Bruer R, Herz U et al. P-and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature 1997; 385: 81–83.
Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M . Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest 2000; 105: 1289–1298.
Sun Y, Tawara I, Toubai T, Reddy P . Pathophysiology of acute graft-versus-host disease: recent advances. Transl Res 2007; 150: 197–214.
Tawara I, Maeda Y, Sun Y, Lowler KP, Liu C, Toubai T et al. Combined Th2 cytokine deficiency in donor T cells aggravates experimental acute graft versus host disease. Exp Hematol 2008; 36: 988–996.
Brok HP, Heidt PJ, van der Meide PH, Zurcher C, Vossen JM . Interferon-gamma prevents graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Immunol 1993; 151: 6451–6459.
Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M . Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest 1998; 102: 2126–2135.
Murphy WJ, Welniak LA, Taub DD, Wiltrout RH, Taylor PA, Vallera DA et al. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest 1998; 102: 1742–1748.
Acknowledgements
We are grateful to Miao Chen, Qiangguo Gao and Yiqi Liu (Second Military Medical University, Shanghai, China) for technical support and offer special thanks to Professor Qing Yi (M.D. Anderson Cancer Center; Houston, TX, USA) for helpful guidance in the experiments. We thank Shizuo Akira (Osaka University, Osaka, Japan) for originally providing key mouse strains. This work was supported by grants of the National Natural Science Foundation of China (no. 30772502 and 30973455), Zhejiang Major Medical and the Health Science and Technology & Ministry of Health of the Chinese Government (no. WKJ2009-2-022). This work was also supported by the Major Research Plan of the Chinese National Natural Science Foundation (no. 91029740), Zhejiang Province Science and Technology Department Foundation (no. 2009C03012-2) and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhao, Y., Liu, Q., Yang, L. et al. TLR4 inactivation protects from graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Cell Mol Immunol 10, 165–175 (2013). https://doi.org/10.1038/cmi.2012.58
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2012.58
Keywords
This article is cited by
-
Viral inhibitory potential of hyoscyamine in Japanese encephalitis virus–infected embryonated chicken eggs involving multiple signaling pathways
Archives of Virology (2023)
-
Challenges and opportunities targeting mechanisms of epithelial injury and recovery in acute intestinal graft-versus-host disease
Mucosal Immunology (2022)
-
Lipopolysaccharide mediates immuno-pathological alterations in young chicken liver through TLR4 signaling
BMC Immunology (2017)
-
Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease
Mucosal Immunology (2015)