Abstract
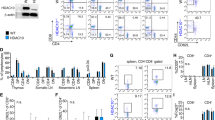
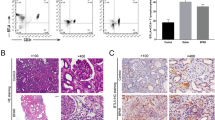
Suberoylanilide hydroxamic acid (SAHA), as a histone deacetylase (HDAC) inhibitor (HDACi), was recently found to exhibit an immunosuppressive effect. However, whether SAHA can synergize with calcineurin inhibitors (CNIs) to inhibit allograft rejection and its underlying mechanism remain elusive. In this study, we demonstrated the synergistic effects of SAHA and non-therapeutic dose of tacrolimus (FK506) in prolonging the allograft survival in a murine cardiac transplant model. Concomitant intragraft examination revealed that allografts from SAHA-treated recipients showed significantly lower levels of IL-17 expression, and no discernable difference for IL-17 expressions was detected between SAHA- and SAHA/FK506-treated allograft as compared with allografts from FK506-treated animals. In contrast, administration of FK506 significantly suppressed interferon (IFN)-γ but increased IL-10 expression as compared with that of SAHA-treated animals, and this effect was independent of SAHA. Interestingly, SAHA synergizes with FK506 to promote Foxp3 and CTLA4 expression. In vitro, SAHA reduced the proportion of Th17 cells in isolated CD4+ T-cell population and decreased expressions of IL-17A, IL-17F, STAT3 and RORγt in these cells. Moreover, SAHA enhances suppressive function of regulatory T (Treg) cells by upregulating the expression of CTLA-4 without affecting T effector cell proliferation, and increased the proportion of Treg by selectively promoting apoptosis of T effector cells. Therefore, SAHA, a HDACi, may be a promising immunosuppressive agent with potential benefit in conjunction with CNI drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nankivell BJ, Kuypers DR . Diagnosis and prevention of chronic kidney allograft loss. Lancet 2011; 378: 1428–1437.
Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D . Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 2000; 342: 605–612.
Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB . Strategies to improve long-term outcomes after renal transplantation. N Engl J Med 2002; 346: 580–590.
Casey MJ, Meier-Kriesche HU . Calcineurin inhibitors in kidney transplantation: friend or foe? Curr Opin Nephrol Hypertens 2011; 20: 610–615.
van de Wetering J, Koumoutsakos P, Peeters A, van der Mast BJ, de Kuiper P, Ijzermans JN et al. Discontinuation of calcineurin inhibitors treatment allows the development of FOXP3+ regulatory T-cells in patients after kidney transplantation. Clin Transplant 2011; 25: 40–46.
Li Y, Shi Y, Huang Z, Bai Y, Niu Q, Cai B et al. CNI induced Th17/Treg imbalance and susceptibility to renal dysfunction in renal transplantation. Int Immunopharmacol 2011; 11: 2033–2038.
Ponticelli C, Scolari MP . Calcineurin inhibitors in renal transplantation still needed but in reduced doses: a review. Transplant Proc 2010; 42: 2205–2208.
Zeng C, Shi X, Zhang B, Liu H, Zhang L, Ding W et al. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl) 2011; 90: 175–186.
Shen Y, Tang XY, Yang YC, Ke X, Kou W, Pan CK et al. Impaired balance of Th17/Treg in patients with nasal polyposis. Scand J Immunol 2011; 74: 176–185.
Niu Q, Cai B, Huang ZC, Shi YY, Wang LL . Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol Int 2011; in press.
Hanidziar D, Koulmanda M . Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr Opin Organ Transplant 2010; 15: 411–415.
Wood KJ . Regulatory T cells in transplantation. Transplant Proc 2011; 43: 2135–2136.
Abadja F, Sarraj B, Ansari MJ . Significance of T helper 17 immunity in transplantation. Curr Opin Organ Transplant 2012; 17: 8–14.
Shahbazian MD, Grunstein M . Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 2007; 76: 75–100.
Ropero S, Esteller M . The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 2007; 1: 19–25.
Minucci S, Pelicci PG . Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006; 6: 38–51.
Yang XJ, Seto E . HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007; 26: 5310–5318.
Hake SB, Xiao A, Allis CD . Linking the epigenetic ‘language’ of covalent histone modifications to cancer. Br J Cancer 2007; 96 Suppl: R31–R39.
Singh BN, Zhang G, Hwa YL, Li J, Dowdy SC, Jiang SW . Nonhistone protein acetylation as cancer therapy targets. Expert Rev Anticancer Ther 2010; 10: 935–954.
Roger T, Lugrin J, Le Roy D, Goy G, Mombelli M, Koessler T et al. Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection. Blood 2011; 117: 1205–1217.
Grabiec AM, Tak PP, Reedquist KA . Function of histone deacetylase inhibitors in inflammation. Crit Rev Immunol 2011; 31: 233–263.
Schildberg FA, Hagmann CA, Bohnert V, Tolba RH . Improved transplantation outcome by epigenetic changes. Transpl Immunol 2010; 23: 104–110.
Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med 2007; 13: 1299–1307.
Moreira JM, Scheipers P, Sorensen P . The histone deacetylase inhibitor Trichostatin A modulates CD4+ T cell responses. BMC Cancer 2003; 3: 30.
Codd R, Braich N, Liu J, Soe CZ, Pakchung AA . Zn(II)-dependent histone deacetylase inhibitors: suberoylanilide hydroxamic acid and trichostatin A. Int J Biochem Cell Biol 2009; 41: 736–739.
Marks PA, Jiang X . Histone deacetylase inhibitors in programmed cell death and cancer therapy. Cell Cycle 2005; 4: 549–551.
Marks PA, Richon VM, Miller T, Kelly WK . Histone deacetylase inhibitors. Adv Cancer Res 2004; 91: 137–168.
Qu W, Kang YD, Zhou MS, Fu LL, Hua ZH, Wang LM . Experimental study on inhibitory effects of histone deacetylase inhibitor MS-275 and TSA on bladder cancer cells. Urol Oncol 2010; 28: 648–654.
Wang Q, Liu Y, Li XK . Simplified technique for heterotopic vascularized cervical heart transplantation in mice. Microsurgery 2005; 25: 76–79.
Xiao L, Fu ZR, Liu F, Zhang LD, Shi XM, Shen XY et al. Suppression of allograft rejection by Tim-1-Fc through cross-linking with a novel Tim-1 binding partner on T cells. PLoS ONE 2011; 6: e21697.
An H, Hou J, Zhou J, Zhao W, Xu H, Zheng Y et al. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat Immunol 2008; 9: 542–550.
Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol 2009; 10: 1252–1259.
Clipstone NA, Crabtree GR . Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 1992; 357: 695–697.
McKinsey TA . The biology and therapeutic implications of HDACs in the heart. Handb Exp Pharmacol 2011; 206: 57–78.
Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005; 352: 1967–1976.
Kinugasa F, Yamada T, Noto T, Matsuoka H, Mori H, Sudo Y et al. Effect of a new immunosuppressant histon deacetylase (HDAC) inhibitor FR276457 in a rat cardiac transplant model. Biol Pharm Bull 2008; 31: 1723–1726.
Wang L, de Zoeten EF, Greene MI, Hancock WW . Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nat Rev Drug Discov 2009; 8: 969–981.
de Zoeten EF, Wang L, Sai H, Dillmann WH, Hancock WW . Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology 2010; 138: 583–594.
Saouaf SJ, Li B, Zhang G, Shen Y, Furuuchi N, Hancock WW et al. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Exp Mol Pathol 2009; 87: 99–104.
de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3+ T-regulatory cells. Mol Cell Biol 2011; 31: 2066–2078.
Moon C, Kim SH, Park KS, Choi BK, Lee HS, Park JB et al. Use of epigenetic modification to induce FOXP3 expression in naive T cells. Transplant Proc 2009; 41: 1848–1854.
Lucas JL, Mirshahpanah P, Haas-Stapleton E, Asadullah K, Zollner TM, Numerof RP . Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol 2009; 257: 97–104.
Johnson J, Pahuja A, Graham M, Hering B, Hancock WW, Bansal-Pakala P . Effects of histone deacetylase inhibitor SAHA on effector and FOXP3+ regulatory T cells in rhesus macaques. Transplant Proc 2008; 40: 459–461.
Liu Z, Zhang C, Sun J . Deacetylase inhibitor trichostatin A down-regulates Foxp3 expression and reduces CD4+CD25+ regulatory T cells. Biochem Biophys Res Commun 2010; 400: 409–412.
Chen N, Gao Q, Field EH . Prevention of Th1 response is critical for tolerance. Transplantation 1996; 61: 1076–1083.
Tay SS, Plain KM, Bishop GA . Role of IL-4 and Th2 responses in allograft rejection and tolerance. Curr Opin Organ Transplant 2009; 14: 16–22.
Korn T, Bettelli E, Oukka M, Kuchroo VK . IL-17 and Th17 Cells. Annu Rev Immunol 2009; 27: 485–517.
Bosisio D, Vulcano M, del Prete A, Sironi M, Salvi V, Salogni L et al. Blocking TH17-polarizing cytokines by histone deacetylase inhibitors in vitro and in vivo. J Leukoc Biol 2008; 84: 1540–1548.
Dixon DL, Griggs KM, Bersten AD, de Pasquale CG . Systemic inflammation and cell activation reflects morbidity in chronic heart failure. Cytokine 2011; 56: 593–599.
Cai Y, Shen X, Ding C, Qi C, Li K, Li X et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 2011; 35: 596–610.
Itoh S, Nakae S, Axtell RC, Velotta JB, Kimura N, Kajiwara N et al. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol 2010; 30: 235–240.
Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol 2007; 179: 4313–4317.
Acknowledgements
This work was supported by grants from the National Key Basic Research Program of China (2009CB522402) and the National Natural Science Foundation of China (31170856, U0832009 and 30772046).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, X., Han, S., Kang, Y. et al. SAHA, an HDAC inhibitor, synergizes with tacrolimus to prevent murine cardiac allograft rejection. Cell Mol Immunol 9, 390–398 (2012). https://doi.org/10.1038/cmi.2012.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2012.28
Keywords
This article is cited by
-
Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Epigenetic alterations are associated with monocyte immune dysfunctions in HIV-1 infection
Scientific Reports (2018)
-
Sirtinol regulates the balance of Th17/Treg to prevent allograft rejection
Cell & Bioscience (2017)