Abstract
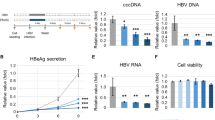
L-ficolin, one of lectin families, is a recently identified complement factor that initiates lectin pathway of complement. Little is known about its role in viral hepatitis. In the present study, we found that L-ficolin in serum from 103 patients with hepatitis C virus (HCV), were significantly higher than that in 150 healthy controls. We further found that L-ficolin expressions were significantly increased in vitro study by HCV JFH-1 infected human hepatocyte cell line Huh7.5.1. Investigation of the mechanisms of the L-ficolin action on HCV demonstrated that L-ficolin protein could recognize and bind to envelope glycoproteins E1 and E2 of HCV, activating the lectin complement pathway-mediated cytolytic activity in HCV-infected hepatocyte. This interaction between L-ficolin and HCV E1 and E2 glycoproteins was attributed to the N-glycans of E1 and E2. These findings provide new insights into the biological functions of L-ficolin in clinically important hepatic viral diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Ali, M., Shi, Y. et al. Specifically Binding of L-ficolin to N-glycans of HCV Envelope Glycoproteins E1 and E2 Leads to Complement Activation. Cell Mol Immunol 6, 235–244 (2009). https://doi.org/10.1038/cmi.2009.32
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2009.32
Keywords
This article is cited by
-
Junctional and somatic hypermutation-induced CX4C motif is critical for the recognition of a highly conserved epitope on HCV E2 by a human broadly neutralizing antibody
Cellular & Molecular Immunology (2021)
-
Ficolin-2 binds to HIV-1 gp120 and blocks viral infection
Virologica Sinica (2016)
-
Serum ficolin-2 concentrations are significantly changed in patients with hepatitis B virus infection and liver diseases
Virologica Sinica (2015)
-
Ficolins and infectious diseases
Virologica Sinica (2014)
-
Ficolin-2 and ficolin-3 in women with malignant and benign ovarian tumours
Cancer Immunology, Immunotherapy (2013)