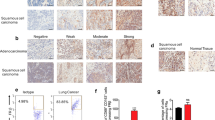
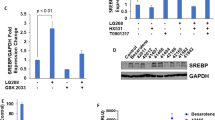
Abstract
The CXCR4 chemokine receptor has an important role in cancer cell metastasis. The CXCR4 antagonist, AMD3100, has limited efficacy in controlling metastasis. HuR, an RNA-binding protein, regulates CXCR4 in cancer cells. We therefore investigated whether targeting HuR using a siRNA-based nanoparticle plus AMD3100 would suppress CXCR4 and inhibit lung cancer metastasis. We treated human H1299 lung cancer cells with HuR-specific siRNA contained in a folate-targeted lipid nanoparticle (HuR-FNP) plus AMD3100, and compared this with AMD3100 alone, HuR-FNP alone and no treatment. HuR-FNP plus AMD3100 treatment produced a G1 phase cell cycle arrest and reduced cell viability above and beyond the effects of AMD3100 alone. HuR and CXCR4 mRNA and protein expression levels were markedly reduced in all treatment groups. Phosphorylated (p) AKTS473 protein was also reduced. P27 protein expression increased with HuR-FNP and combination treatment. Promoter-based reporter studies showed that the combination inhibited CXCR4 promoter activity more than did either treatment alone. Cell migration and invasion was significantly reduced with all treatments; the combination provided the most inhibition. Reduced matrix metalloprotease (MMP)-2 and -9 expression was associated with reduced invasion in all treatment groups. Thus, we found that combined HuR and CXCR4 targeting effectively controlled lung cancer metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D . Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90.
Liu L, Zhao E, Li C, Huang L, Xiao L, Cheng L et al. TRIM28, a new molecular marker predicting metastasis and survival in early-stage non-small cell lung cancer. Cancer Epidemiol 2013; 37: 71–78.
Chaffer CL, Weinberg RA . A perspective on cancer cell metastasis. Science 2011; 331: 1559–1564.
Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Streiter RM et al. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med 2003; 167: 1676–1686.
Vandercappellen J, Van Damme J, Struyf S . The role of CXC chemokines and their receptors in cancer. Cancer Lett 2008; 267: 226–244.
Su L, Zhang J, Xu H, Wang Y, Chu Y, Liu R et al. Differential expression of CXCR4 is associated with the metastatic potential of human non-small cell lung cancer cells. Clin Cancer Res 2005; 11: 8273–8280.
Oonakahara K, Matsuyama W, Higashimoto I, Kawabata M, Arimura K, Osame M et al. Stromal-derived factor-1alpha/CXCL12-CXCR 4 axis is involved in the dissemination of NSCLC cells into pleural space. Am J Respir Cell Mol Biol 2004; 30: 671–677.
Minamiya Y, Saito H, Takahashi N, Ito M, Imai K, Ono T et al. Expression of the chemokine receptor CXCR4 correlates with a favorable prognosis in patients with adenocarcinoma of the lung. Lung Cancer 2010; 68: 466–471.
Wagner PL, Hyjek E, Vazquez MF, Meherally D, Liu YF, Chadwick PA et al. CXCL12 and CXCR4 in adenocarcinoma of the lung: association with metastasis and survival. J Thorac Cardiovasc Surg 2009; 137: 615–621.
Na IK, Scheibenbogen C, Adam C, Stroux A, Ghadjar P, Thiel E et al. Nuclear expression of CXCR4 in tumor cells of non-small cell lung cancer is correlated with lymph node metastasis. Hum Pathol 2008; 39: 1751–1755.
Otsuka S, Klimowicz AC, Kopciuk K, Petrillo SK, Konno M, Hao D et al. CXCR4 overexpression is associated with poor outcome in females diagnosed with stage IV non-small cell lung cancer. J Thorac Oncol 2011; 6: 1169–1178.
Teicher BA, Fricker SP . CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 2010; 16: 2927–2931.
D'Alterio C, Barbieri A, Portella L, Palma G, Polimeno M, Riccio A et al. Inhibition of stromal CXCR4 impairs development of lung metastases. Cancer Immunol Immunother 2012; 61: 1713–1720.
Uchida D, Onoue T, Kuribayashi N, Tomizuka Y, Tamatani T, Nagai H et al. Blockade of CXCR4 in oral squamous cell carcinoma inhibits lymph node metastases. Eur J Cancer 2011; l47: 452–459.
Ramsey DM, McAlpine SR . Halting metastasis through CXCR4 inhibition. Bioorg Med Chem Lett 2013; 23: 20–25.
Uy GL, Rettig MP, Motabi IH, McFarland K, Trinkaus KM, Hladnik LM et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood 2012; 119: 3917–3924.
Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 2009; 113: 6206–6214.
Peled A, Wald O, Burger J . Development of novel CXCR4-based therapeutics. Expert Opin Investig Drugs 2012; 21: 341–353.
Srikantan S, Gorospe M . HuR function in disease. Front Biosci (Landmark Ed) 2012; 17: 189–205.
Abdelmohsen K, Gorospe M . Post transcriptional regulation of cancer traits by HuR. Wiley Interdiscip Rev RNA 2010; 1: 214–229.
Wang J, Guo Y, Chu H, Guan Y, Ji Bi, Wang B . Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis. Int J Mol Sci 2013; 14: 10015–10041.
López de Silanes I, Lal A, Gorospe M . HuR: post-transcriptional paths to malignancy. RNA Biol 2005; 2: 11–13.
Wang WG, Caldwell MC, Lin SK, Furneaux H, Gorospe M . HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J 2000; 19: 2340–2350.
Lauriola L, Granone P, Ramella S, Lanza P, Ranelletti FO . Expression of the RNA-binding protein HuR and its clinical significance in human stage I and II lung adenocarcinoma. Histol Histopathol 2012; 27: 617–626.
Denkert C, Koch I, von Keyserlingk N, Noske A, Niesporek S, Dietel M et al. Expression of the ELAV-like protein HuR in human colon cancer: association with tumor stage and cyclooxygenase-2. Mod Pathol 2006; 19: 1261–1269.
Niesporek S, Kristiansen G, Thoma A, Weichert W, Noske A, Buckendahl AC et al. Expression of the ELAV-like protein HuR in human prostate carcinoma is an indicator of disease relapse and linked to COX-2 expression. Int J Oncol 2008; 32: 341–347.
Wang D, Wang M, Hu C, Shuang T, Zhou Y, Yan X . Expression of the ELAV-like protein HuR in the cytoplasm is associated with endometrial carcinoma progression. Tumour Biol 2014; 35: 11939–11947.
Al-Souhibani N, Al-Ghamdi M, Al-Ahmadi W, Khabar KS . Posttranscriptional control of the chemokine receptor CXCR4 expression in cancer cells. Carcinogenesis 2014; 35: 1983–1992.
Ramesh R, Saeki T, Templeton NS, Ji L, Stephens LC, Ito I et al. Successful treatment of primary and disseminated human lung cancers by systemic delivery of tumor suppressor genes using an improved liposome vector. Mol Ther 2001; 3: 337–350.
Began G, Ito I, Branch CD, Clifton Stephens L, Roth JA, Ramesh R . Nanoparticle based systemic gene therapy for lung cancer: molecular mechanisms, and strategies to suppress nanoparticle-mediated inflammatory response. Technol Cancer Res Treat 2004; 3: 647–657.
Babu A, Wang Q, Muralidharan R, Shanker M, Munshi A, Ramesh R . Chitosan coated poly(lactic acid) polymeric nanoparticle-mediated combinatorial delivery of cisplatin and siRNA/plasmid DNA chemosensitizes cisplatin-resistant human ovarian cancer cells. Mol Pharm 2014; 11: 2720–2733.
Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R . Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Ther 2000; 7: 2051–2057.
Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res 2003; 63: 5105–5113.
Panneerselvam J, Shanker M, Jin J, Branch CD, Muralidharan R, Zhao DY et al. Phosphorylation of interleukin (IL)-24 is required for mediating its anti-cancer activity. Oncotarget 2015; 18: 16271–16286.
Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S . Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol Ther 2004; 9: 510–518.
Panneerselvam J, Jin J, Shanker M, Lauderdale J, Bates J, Wang Q et al. Disruption of the CXCR-4/SDF axis by interleukin (IL)-24 reduces tumor cell migration and invasion in lung cancer cells. PLoS One 2015; 10: e0122439.
Liu J, Ping W, Zu Y, Sun W . Correlations of lysyl oxidase with MMP2/MMP9 expression and its prognostic value in non-small cell lung cancer. Int J Clin Exp Pathol 2014; 7: 6040–6047.
Pritchard SC, Nicolson MC, Lloret C, McKay JA, Ross VG, Kerr KM et al. Expression of matrix metalloproteinases 1, 2, 9 and their tissue inhibitors in stage II non-small cell lung cancer: implications for MMP inhibition therapy. Oncol Rep 2001; 8: 421–424.
Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J 2004; 18: 1240–1242.
Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD et al. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate 2006; 66: 32–48.
Muralidharan R, Babu A, Basalingappa K, Mehta M, Munshi A, Ramesh R . Designing of tumor-targeted HuRsiRNA nanoparticle as a therapeutic for lung cancer. In: Gandhi V, Mehta K, Grover RK, Pathak S, Aggarwal BB (eds). Multitargeted Approach to the Treatment of Cancer. Springer International Publishing: Switzerland, pp 277–294, 2015.
Babu A, Amreddy N, Muralidharan R, Munshi A, Ramesh R Nanoparticle-mediated siRNA delivery for lung cancer treatment. In: Naik J (ed). Nano Based Drug Delivery. IAPC Publishing: Croatia, pp 195–215, 2015.
Akool El-S, Kleinert H, Hamada FM, Abdelwahab MH, Förstermann U, Pfeilschifter J et al. Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR. Mol Cell Biol 2003; 23: 4901–4916.
Chen H, Xu X, Teng J, Cheng S, Bunjhoo H, Cao Y et al. CXCR4 inhibitor attenuates allergen-induced lung inflammation by down-regulating MMP-9 and ERK1/2. Int J Clin Exp Pathol 2015; 8: 6700–6777.
Acknowledgements
The study was supported in part by a grant received from the National Institutes of Health (NIH) R01 CA167516, an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (P20 GM103639) of the National Institutes of Health, and by funds received from the Jim and Christy Everest Endowed Chair in Cancer Developmental Therapeutics, the University of Oklahoma Health Sciences Center. We thank the Stephenson Cancer Center at the University of Oklahoma Health Sciences Center, Oklahoma City, OK, for the use of the cancer Functional Genomics Core and Biostatistics Core, which provided molecular analysis and statistical services. We thank Ms. Kathy Kyler at the office of Vice President of Research, OUHSC for editorial assistance. Rajagopal Ramesh is an Oklahoma TSET Research Scholar and holds the Jim and Christy Everest Endowed Chair in Cancer Developmental Therapeutics.
Author contributions
RM and JP conducted the experiments. RM synthesized and characterized the nanoparticle (HuR-FNP and C-FNP). RM, JP, AC, YDZ, AM and RR conceived and designed the experiments. RM and RR wrote the paper. AC and YDZ performed statistical analysis. Together, RM, JP, AC, YDZ, AM and RR did data collection, analysis and interpretation. RM, JP, AC, YDZ, AM and RR critically reviewed, revised for intellectual content, and provided suggestions. RR supervised the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Muralidharan, R., Panneerselvam, J., Chen, A. et al. HuR-targeted nanotherapy in combination with AMD3100 suppresses CXCR4 expression, cell growth, migration and invasion in lung cancer. Cancer Gene Ther 22, 581–590 (2015). https://doi.org/10.1038/cgt.2015.55
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2015.55
This article is cited by
-
DDIAS, DNA damage-induced apoptosis suppressor, is a potential therapeutic target in cancer
Experimental & Molecular Medicine (2023)
-
Crabp2 Promotes Metastasis of Lung Cancer Cells via HuR and Integrin β1/FAK/ERK Signaling
Scientific Reports (2019)
-
Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA
Cell Death & Differentiation (2019)
-
Quantitative characterization of mechano-biological interrelationships of single cells
The International Journal of Advanced Manufacturing Technology (2019)
-
Targeting the CXCR4/CXCL12 axis in treating epithelial ovarian cancer
Gene Therapy (2017)