Abstract
Epithelial–mesenchymal transition (EMT) is a key process in the tumor metastatic cascade that is characterized by the loss of cell–cell junctions and cell polarity, resulting in the acquisition of migratory and invasive properties. Recent evidence showed that altered microRNA-10b (miR-10b) expression was implicated in the occurrence of EMT of breast cancer. However, the exact role and underlying mechanisms of miR-10b in the EMT of breast cancer still remain unknown. In this study, miR-10b was found to be upregulated in breast cancer tissues and breast cancer cell lines and the expression of miR-10b was shown to be closely correlated with aggressiveness in breast cancer. Treating breast cancer cells with the miR-10b inhibitor increased E-cadherin expression while decreasing vimentin expression. At the same time, on inhibition of miR-10b, the invasion and proliferation ability of breast cancer cells also decreased. Transforming growth factor-β (TGF-β) is a multifunctional cytokine that induces EMT in multiple cell types. Here, we identified miR-10b as a target gene of TGF-β1. The expression of miR-10b increased during TGF-β1-induced EMT of breast cancer cells. Further study showed that inhibition of miR-10b expression partially reversed the EMT, invasion and proliferation induced by TGF-β1 in breast cancer cells. Taken together, these results demonstrated a novel function for miR-10b in TGF-β1-induced EMT in breast cancer and increased their metastatic potential. MiR-10b might become a possible target for gene therapy in breast cancer.
Similar content being viewed by others
Main
Breast cancer is one of the most common types of malignant cancers worldwide. Even though there has been considerable progress in the early detection and surgical therapy of breast cancer, there are ∼350 000 women who die from breast cancer each year.1 The principal reason for mortality in breast cancer is invasion and metastasis rather than the primary cancer itself; therefore, there is an urgent need to understand the molecular mechanism and pathways that participate in the invasion and metastasis of breast cancer for better and improved treatment of women diagnosed with breast cancer.2 Epithelial–mesenchymal transition (EMT) is a key step toward cancer metastasis, and E-cadherin is regarded as a main indicator of the epithelial–mesenchymal phenotype switching.3 E-cadherin loss is suggestive of EMT, and tumor cell invasion and metastasis are associated with EMT.4, 5
EMT is triggered by many signaling pathways—for example, transforming growth factor-β (TGF-β),6 fibroblast growth factor,7 epidermal growth factor,8 hepatocyte growth factor,9 platelet-derived growth factors10 as well as different isoforms of Wnt proteins,11 matrix metalloproteinases,12 bone morphogenic proteins13 and many others. Among these signaling pathways, TGF-β has been claimed to be critical for induction of the EMT phenotype, and TGF-β as a potent inducer of EMT was first recognized in cultured normal mammary epithelial cells.14 The functioning of the TGF-β pathway depends on its constitutive and extensive communication with other genes, resulting in synergistic or antagonistic effects and desirable biological outcomes.15
MicroRNAs (miRNAs) are emerging regulators of gene expression at the post-transcriptional level. Emerging evidence shows that miRNAs are involved in the biological processes related to apoptosis, proliferation, differentiation, invasion and metastasis, while its deregulation is crucial to cancer initiation and progression.16, 17, 18, 19 In an initial screen for miRNAs differentially expressed in human breast cancer cells, the three most significantly upregulated miRNAs, miR-155, miR-9 and microRNA-10b (miR-10b), were identified.20 MiR-10b is a particularly interesting candidate given its close correlation with metastatic behaviors.21 Ma et al.22 reported that overexpression of miR-10b could endow breast cancer cells with invasive and metastatic abilities in vivo. MiR-10b is also involved in the progression of other types of cancer. Antisense silencing of miR-10b in NF1 malignant peripheral nerve sheath tumor cells reduced cell proliferation, migration and invasion. Recent reports identified the miR-10b gene as a target of the transcription factor Twist, which is highly expressed in metastatic breast cancer cells and stimulates in vitro and in vivo tumor invasion.23 However, its role in the occurrence of EMT has not been fully elucidated.
Here we demonstrated a novel link between miR-10b activation and the TGF-β1 signaling pathway. We showed that miR-10b was overexpressed in breast cancer tissue and breast cancer cells and the expression of miR-10b was shown to be closely correlated with aggressiveness in breast cancer. Inhibition of miR-23a could suppress the occurrence of EMT and the invasion and proliferation of breast cancer. Furthermore, inhibition of miR-10b could also partially suppress the EMT, invasion and proliferation induced by TGF-β1 in breast cancer cells. MiR-10b could be a useful target for breast cancer prevention and therapy.
Material and methods
Ethics statement
This study was performed under a protocol approved by the Institutional Review Boards of The First Affiliated Hospital of Zhengzhou Medical University, and all examinations were performed after obtaining written informed consent.
Tissue specimens
Thirty-four tumor and adjacent normal tissue samples were collected from breast cancer patients undergoing surgical resection at First Affiliated Hospital of Zhengzhou University. A board certified pathologist diagnosed all tumor tissue as breast cancer. Normal samples were collected from areas adjacent to the tumor tissue but outside the tumor margins. RNA was isolated from fresh frozen tissue samples using TRIzol reagent. The isolated RNA was dissolved in RNase-free water and stored at −70 °C.
Cell line and cell culture
The human breast cancer cell lines MCF-7, MDA-MB-231 and MDA-MB-435 were cultured in RPMI-1640 medium with 10% fetal bovine serum. The cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. All of these cells were purchased from a commercial company within 4 years. MDA-MB-231 and MDA-MB-435 cell lines are highly invasive human breast cancer cell lines, whereas the MCF-7 cell line is a well-accepted human breast cancer cell line with relatively low invasiveness.
Transfection
Inhibitors for miR-10b and control oligonucleotide were purchased from Shanghai GenePharma (Shanghai, China). The concentration used was 50 nM. Breast cancer cells were seeded 24 h before transfection in 6-well plates and transfected using Lipofectamine 2000 transfection reagent in accordance with the manufacturer’s advised procedure. The efficiency of miR-10b inhibiting was evaluated with quantitative real-time PCR tests. In other sets of experiments, TGF-β1 was purchased from Aviscera Bioscience (Santa Clara, CA, USA) and the concentration used was 5 ng ml−1 for 48 h.
RNA and miRNA isolation and cDNA synthesis, quantitative real-time PCR of miR-10b, E-cadherin and vimentin
Total RNA from breast cancer tissues and cells was isolated using Trizol reagents and miRNA was isolated with a mirVana miRNA isolation Kit. The quality and quantity of the RNA and miRNA samples were assessed by standard electrophoresis and spectrophotometric methods. cDNA from total RNA was generated using a TransScript First-strand cDNA Synthesis SuperMix kit. cDNA from miRNAs was generated using a TaqMan miRNA Reverse Transcription kit. The expression level of E-cadherin and vimentin was measured by quantitative real-time PCR using the SYBR Premix Ex Taq kit with the following primers: E-cadherin forward 5′-gaacgcattgccacatacac-3′ and E-cadherin reverse 5′-attcgggcttgttgtcattc-3′; Vimentin forward 5′-gagaactttgccgttgaagc-3′ and vimentin reverse 5′-ctcaatgtcaagggccatct-3′, and normalized using β-actin. The expression level of miR-21 was measured by quantitative real-time PCR using the TaqMan MicroRNA Assay protocol specific for miR-10b and normalized using U6 small nuclear RNA (RNU6B). All of the reactions were run in triplicate. The ΔΔCt method for relative quantification of gene expression was used to determine miR-10 and E-cadherin and vimentin mRNA expression levels.
Western blot analysis
Experimental cells were harvested and lysed for total protein extraction. Protein content in cell lysates was measured using the Micro BCA protein assay kit (Pierce Biotechnology/Thermo Fisher Scientific, Rockford, IL, USA) using bovine serum albumin as the internal standard. Fifty micrograms of total protein from experimental cells was separated on a 10% Tris-glycine SDS polyacrylamide gel and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The blot was probed with mouse monoclonal antibodies including anti-β-Actin (Santa Cruz, CA, USA; 1:5000), anti-E-cadherin (Santa Cruz; 1:2000) and anti-vimentin (Santa Cruz; 1:2000). Antibody binding was detected using Gel Blot Imaging Systems (Syngene G:BOX, Cambridge, UK) according to the manufacturer’s protocol. The expression of β-actin was used as a normalization control for protein loading.
Cell proliferation assay
Cell proliferation was monitored by the colorimetric water-soluble tetrazolium salt (CCK8) assay using Cell Counting Kit-8 (Dojindo, Shanghai, China) according to the manufacturer’s instructions. Breast cancer cells were seeded onto 96-well plates (2 × 103 cells per well), and cell proliferation was documented every 24 h for 3 days. The staining intensity in the medium was measured by determining the absorbance at 450 nm. The results were representative of three individual experiments in triplicate.
Invasion assay
The invasion of breast cancer cells was measured using Boyden transwell chambers (Transwell-Costar, Corning, NY, USA) according to the manufacturer's protocol. Briefly, the cells were seeded onto the membrane of the upper chamber at a concentration of 3 × 104 in 400 μL medium, and RPMI-1640 medium was added to the lower chamber. After 24 h, the lower chamber was washed three times with deionized water. The membrane in the lower chamber was stained with crystal violet. The number of invaded cells was counted with a microscope in at least three fields.
Statistical analysis
The database was set up with the SPSS 11.0 software package (Selles, Zhengzhou, China) for analysis. Data were presented as mean±s.d. The mean values of multiple groups were compared with one-way analysis of variance after the equal check of variance, and the two-way comparisons among the means were performed using the least significant difference method. Statistical comparison was also performed with the two-tailed t-test when appropriate. P<0.05 was considered statistically significant.
Results
The expression of miR-10b was upregulated in breast cancer tissues and cells
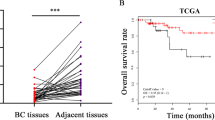
We measured miR-10b expression levels in 34 frozen samples from breast cancer patients (17 tumor samples and 17 adjacent normal control samples) using quantitative real-time PCR. Our results showed that miR-10b expression was significantly upregulated in breast cancer tissue as compared with adjacent normal tissue (Figure 1a). Furthermore, quantitative real-time PCR was also performed to detect the miR-10b expression level in breast cancer cells in order to validate the high-expression trend of miR-10b in the breast cancer tissues. We found that miR-10b was expressed in all three breast cancer cell lines (Figure 1b). More importantly, a correlation was found to exist between the aggressiveness of the cell line and expression of miR-10b in all three breast cancer cell lines. MDA-MB-231 and MDA-MB-435 with high metastatic ability revealed a higher miR-10b expression, whereas the poorly metastatic breast cancer cell line MCF-7 revealed a lower miR-10b expression.
Expression of microRNA-10b (miR-10b) was upregulated in breast cancer tissues and breast cancer cell lines. (a) MiR-10b expression was examined in 34 cases of frozen samples from breast cancer patients (17 tumors and 17 adjacent normal controls) using quantitative real-time PCR. The expression of miR-10b in breast cancer tissues was significantly higher than that in adjacent normal controls, *P<0.01. (b) MiR-10b expression was analyzed in three cancer cell lines (MCF-7, MDA-MB-231 and MDA-MB-435) using quantitative real-time PCR. The expression of miR-10b in breast cancer cell lines MDA-MB-231 and MDA-MB-435 was higher than that in breast cancer cell line MCF-7, *P<0.05.
Effect of miR-10b inhibition on invasion and proliferation ability of breast cancer cells
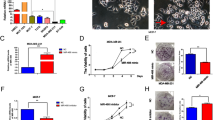
Next, we examined the role of miR-10b in breast cancer invasion and proliferation using two kinds of breast cancer cell lines, MDA-MB-231 and MDA-MB-435, which had higher miR-10b expression. Fifty nanomolars of miR-10b inhibitor was used to transfect MDA-MB-231 and MDA-MB-435 cells. MDA-MB-435 and MDA-MB-231 cells transiently transfected with control oligonucleotides were used as negative control, and blank MDA-MB-231 and MDA-MB-435 cells were used as blank control. At 48 h after transfection, miR-10b expression in both types of breast cancer cells showed a markedly downregulated miR-10b expression compared with that in negative control or blank control (Figure 2a). As previously mentioned, metastasis is an important characteristic of malignant cancer cells. To further assess the influence of miR-10b on breast cancer cell invasion, the effect of miR-10b inhibition on cell invasion ability was examined in MDA-MB-231 and MDA-MB-435 cells. The Boyden chamber invasion assay showed that inhibition of miR-10b significantly decreased the invasiveness of MDA-MB-231 and MDA-MB-435 cells (Figures 2b and c). These data indicated that miR-10b might be a key factor stimulating the invasion of breast cancer cells. To ascertain whether the effects were truly invasive, we needed to show the effect of inhibiting miR-10b on the proliferation ability of MDA-MB-231 and MDA-MB-435 cells. Hence, the proliferation ability change of MDA-MB-231 and MDA-MB-435 after transfection with miR-10b inhibitor at different time intervals (24, 48 and 72 h) was measured by the CCK8 assay. Compared with negative control and blank control, MDA-MB-231 and MDA-MB-435 cells transfected with miR-10b inhibitor showed significantly decreased optical density values at 48 and 72 h, respectively (Figure 2d). These results provided the evidence that miR-10b expression may have a role in breast cancer invasion and proliferation.
MicroRNA-10b (MiR-10b) inhibited the invasion and proliferation of breast cancer cell lines MDA-MB-231 and MDA-MB-435. (a) Relative miR-10b expression levels in three groups of MDA-MB-231 and MDA-MB-435 cells (Blank: MDA-MB-231 and MDA-MB-435; negative: MDA-MB-231 and MDA-MB-435 transfected with control oligonucleotides; miR-10b inhibitor: MDA-MB-231 and MDA-MB-435 transfected with 50 nM miR-10b inhibitor for 24 h). U6 small nuclear RNA (RNU6B) was used as an internal loading control to normalize the results. The miR-10b expression in MDA-MB-231 and MDA-MB-435 cells transfected with miR-10b inhibitor was significantly lower than that in blank control and negative control, *P<0.01. (b) Representative photographs of invaded MDA-MB-231 and MDA-MB-435 cells on the membrane at a magnification of × 100. (c) Quantitative results for the invasion ability of each group of MDA-MB-231 and MDA-MB-435 cells were shown as invaded cell number, 24 h after incubation. The average cell counts of MDA-MB-231 and MDA-MB-435 cells transiently transfected with miR-10b inhibitor invading through the membrane were smaller than those of negative control or blank control, *P<0.05. (d) Every 24 h, CCK8 assay was performed on three groups of MDA-MB-231 and MDA-MB-435 cells each. The viable cell number was evaluated as the value of the absorbance at 450 nm. The optical density (OD) values of MDA-MB-231 and MDA-MB-435 cells transfected with 50 nM miR-10b inhibitor for 24 h was smaller than that in control groups, *P<0.05, **P<0.01.
Effect of miR-10b inhibition on the expression of E-cadherin and vimentin
A series of mechanisms are involved in the invasion of breast cancer, and EMT has a particularly critical role. E-cadherin downregulation in mammalian cell systems is sufficient to trigger EMT.24 Gupta et al.25 have reported that, in colorectal carcinomas, the embryonic EMT is activated during tumor invasion in disseminating cancer cells. These cells are characterized by a loss of E-cadherin expression. We detected the expression of epithelial marker (E-cadherin) and mesenchymal marker (vimentin) of cells treated with miR-10b inhibitor to ascertain the effects of miR-10b on EMT. Given that the treatment with the miR-10b inhibitor resulted in a reduction in miR-10b expression level, we analyzed the mRNA and protein expression of E-cadherin and vimentin in MDA-MB-231 and MDA-MB-435 cells with reduced miR-10b levels. As shown in Figure 3a, miR-10b inhibition induced an increase in expression of E-cadherin and a decrease in expression of vimentin relative to that in controls. The data from western blot of E-cadherin and vimentin showed results similar to those of quantitative real-time PCR (Figure 3b).
MicroRNA-10b (MiR-10b) altered messenger RNA (mRNA) and protein expression of E-cadherin and vimentin in breast cancer cell lines MDA-MB-231 and MDA-MB-435. (a) The mRNA levels of E-cadherin and vimentin in three groups of MDA-MB-231 and MDA-MB-435 cells, respectively. (Blank: MDA-MB-231 and MDA-MB-435; negative: MDA-MB-231 and MDA-MB-435 transfected with control oligonucleotides; miR-10b inhibitor: MDA-MB-231 and MDA-MB-435 transfected with 50 nM miR-10b inhibitor for 24 h). β-actin was used as an internal control. The E-cadherin mRNA expression in MDA-MB-231 and MDA-MB-435 cells transfected with miR-10b inhibitor was significantly higher than that in blank control and negative control (P<0.05), whereas vimentin mRNA expression in MDA-MB-231 and MDA-MB-435 cells transfected with miR-10b inhibitor was significantly lower than that in blank control and negative control, *P<0.05. (b) The protein levels of E-cadherin and vimentin in three groups of MDA-MB-231 and MDA-MB-435 cells, respectively. β-actin was used as an internal control. The E-cadherin protein expression in MDA-MB-231 and MDA-MB-435 transfected with miR-10b inhibitor was significantly higher than that in blank control and negative control (P<0.05), whereas vimentin protein expression in MDA-MB-231 and MDA-MB-435 cells transfected with miR-10b inhibitor was significantly lower than that in blank control and negative control, *P<0.05.
MiR-10b expression increased during TGF-β1-induced EMT in breast cancer cell lines
Transforming growth factor-β (TGF-β) has a central role in the regulation of EMT. TGF-β is often overexpressed in tumor tissues including breast cancer, and facilitates cancer progression through a diverse repertoire of tumor-cell-autonomous and host–tumor interactions, including enhancement of cell motility and invasion, which involves the process of EMT.26, 27 When stimulated by 5 ng ml−1 TGF-β1 for 24 h, breast cancer cells MDA-MB-231 and MDA-MB-435 undergo EMT by showing less uniform epithelial morphological changes, which were correlated with decreased E-cadherin expression and increased vimentin expression (Figures 4a and b). miRNAs are already known to be key regulators of the TGF-β pathway.28 In order to study whether miR-10b was involved in the TGF-β1 signaling pathway in breast cancer, MDA-MB-231 and MDA-MB-435 cells were used again. Quantitative real-time PCR demonstrated that TGF-β1, but not the control, induced miR-10b expression significantly in MDA-MB-231 and MDA-MB-435 cells (Figure 4c). All these results imply that TGF-β signaling is necessary for miR-10b expression.
MicroRNA-10b (MiR-10b) was directly induced by TGF-β1 and involved in the EMT of breast cancer cells. (a) The messenger RNA (mRNA) levels of E-cadherin and vimentin in two groups of MDA-MB-231 and MDA-MB-435 cells, respectively (TGF-β1: MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h; control: MDA-MB-231 and MDA-MB-435 cells). β-actin was used as an internal control. Compared with vacant MDA-MB-231 and MDA-MB-435 cells, loss of E-cadherin expression and enhanced vimentin expression were observed in MDA-MB-231 and MDA-MB-435 cells after TGF-β1 stimulation,*P<0.05.(b) The protein levels of E-cadherin and vimentin in two groups of MDA-MB-231 and MDA-MB-435 cells. Compared with vacant MDA-MB-231 and MDA-MB-435 cells, loss of E-cadherin expression and enhanced vimentin expression were observed in MDA-MB-231 and MDA-MB-435 cells after TGF-β1 stimulation, *P<0.05. (c) The miR-10b expression in two groups of MDA-MB-231 and MDA-MB-435 cells. U6 small nuclear RNA (RNU6B) was used as an internal loading control to normalize the results. Compared with vacant MDA-MB-231 and MDA-MB-435 cells, high miR-10b expression was observed in MDA-MB-231 and MDA-MB-435 cells after TGF-β1 stimulation, *P<0.01.
MiR-10b inhibitor rescued TGF-β1-induced EMT in breast cancer cell lines
To further investigate the role of miR-10b in TGF-β1-induced EMT, we used the miR-10b inhibitor to determine the effect of miR-10b blockade on the TGF-β 1 signaling pathway and on the EMT process. MDA-MB-231 and MDA-MB-435 cells were incubated with 5 ng ml−1 TGF-β1 for 24 h and then transfected with 2 uM miR-10b inhibitor for 48 h. TGF-β1 stimulation decreased E-cadherin expression and enhanced vimentin expression, whereas the miR-10b inhibitor reversed the expression of E-cadherin and vimentin (Figures 5a and b). Thus, our results suggest that miR-10b has an important role in TGF-β1-induced EMT in breast cancer.
Inhibition of microRNA-10 (miR-10) could partially restore the epithelial–mesenchymal transition of breast cancer cells induced by transforming growth factor-β1 (TGF-β1). (a) The messenger RNA (mRNA) levels of E-cadherin and vimentin in four groups of MDA-MB-231 and MDA-MB-435 cells (TGF-β1: MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h; miR-10b inhibitor: MDA-MB-231 and MDA-MB-435 cells transfected with 50 nM miR-10b inhibitor for 24 h; TGF-β1+miR-10b inhibitor: MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h then transfected with 50 nM miR-10b inhibitor for 24 h; control: MDA-MB-231 and MDA-MB-435 cells). β-actin was used as an internal control. Compared with MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h, MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h and then transfected with 50 nM miR-10b inhibitor for 24 h showed altered E-cadherin and vimentin mRNA expression, *P<0.05. (b) The protein levels of E-cadherin and vimentin in four groups of MDA-MB-231 and MDA-MB-435 cells. Compared with MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h, MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h and then transfected with 50 nM miR-10b inhibitor for 24 h showed altered E-cadherin and vimentin protein expression, *P<0.05.
MiR-10b inhibitor rescued TGF-β1-induced higher invasion and proliferation ability of breast cancer cell lines
Next, we determined whether miR-10b blockade affected the invasion and proliferation ability of MDA-MB-231 and MDA-MB-435 cells after treatment with 5 ng/ml TGF-β1 for 24 h. The results of boyden chamber and CCK8 showed that TGF-β1 stimulation could increase the invasion and proliferation ability of MDA-MB-231 and MDA-MB-435 cells, but miR-10b blockade reversed the stimulatory effect of TGF-β1 on cell invasion and proliferation of MDA-MB-231 and MDA-MB-435 cells (Figures 6a and b), suggesting that miR-10b inhibition altered the TGF-β1 signaling pathway in breast cancer cells, affecting cell invasion and cell proliferation.
Inhibition of microRNA-10 (miR-10) could partially restore the invasion and proliferation ability of breast cancer cells induced by transforming growth factor-β (TGF-β1). (a) Quantitative results for the invasion ability of four groups of MDA-MB-231 and MDA-MB-435 cells were shown as invaded cell number, 24 h after incubation (TGF-β1: MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h; miR-10b inhibitor: MDA-MB-231 and MDA-MB-435 cells transfected with 50 nM miR-10b inhibitor for 24 h; TGF-β1+ miR-10b inhibitor: MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h then transfected with 50 nM miR-10b inhibitor for 24 h; control: MDA-MB-231 and MDA-MB-435 cells). The average cell counts of MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h and then transfected with 50 nM miR-10b inhibitor for 24 h invading through the membrane were smaller than those of MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h, *P<0.05. (b) Every 24 h, the CCK8 assay was performed on four groups of MDA-MB-231 and MDA-MB-435 cells. The viable cell number was evaluated as the value of the absorbance at 490 nm. The optical density (OD) values of MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h and then transfected with 50 nM miR-10b inhibitor for 24 h invading through the membrane were smaller than those of MDA-MB-231 and MDA-MB-435 cells treated with 5 ng ml−1 of TGF-β1 for 24 h, *P<0.05.
Discussion
Metastasis is the major cause of breast cancer mortality. Identification of metastasis-associated mechanisms is essential for developing new markers for early detection, and ultimately discovering new therapeutic targets. EMT is a normal physiological process that is essential for embryogenesis and tissue morphogenesis and for tissue remodeling and repair during wound healing.4, 29, 30 However, pathological EMT is increasingly recognized to have an important role during the development of human diseases, including chronic inflammation, fibrosis, rheumatoid arthritis and cancer invasion and metastasis.3, 31, 32 miRNAs are endogenous small non-coding RNAs that control the target gene expression at the post-transcriptional level. Several miRNAs have been functionally classified as proto-oncogenes or tumor suppressors and are aberrantly expressed in different cancer types.16, 33 Previous studies have suggested that deregulation of miR-10b is associated with EMT and the progression of breast cancer.34 However, the involvement of miR-10b in the EMT of breast cancer has not been investigated fully. In the current study, quantitative real-time PCR analysis confirmed that breast cancer exhibits abundant miR-10b expression, in contrast to adjacent non-tumor tissues, which displayed absence or lower miR-10b expression. We also showed that miR-10b expression determined by quantitative real-time PCR was expressed in all studied breast cancer cell lines (MCF-7, MDA-MB-231 and MDA-MB-435). More importantly, the expression of miR-10b in MCF-7, MDA-MB-231 and MDA-MB-435 cells was found to be positively correlated with the aggressiveness of the cell line: MDA-MB-231 and MDA-MB-435 cell lines with higher metastatic ability revealed higher miR-10b expression, whereas the poorly metastatic MCF-7 cell line showed lower miR-10b expression.
To further confirm the role of miR-10b in the invasion and metastasis of ESCC, we selected MDA-MB-231 and MDA-MB-435 cells with a relatively higher miR-10b expression for further study. We found that the inhibition of miR-10b could suppress the occurrence of EMT in MDA-MB-231 and MDA-MB-435 cells significantly, accompanied by the declined invasion and proliferation properties of MDA-MB-231 and MDA-MB-435 cells. EMT is marked by the loss of epithelial markers, such as E-cadherin, and the acquisition of mesenchymal markers, such as vimentin.35 Our data demonstrated that the expression of E-cadherin was elevated significantly in MDA-MB-231 and MDA-MB-435 cells transfected with the miR-10b inhibitor compared with control groups. In contrast, the expression of vimentin was decreased greatly in MDA-MB-231 and MDA-MB-435 cells transfected with the miR-10b inhibitor. Furthermore, our data also showed that miR-10b was a major regulator of breast cancer cell invasion. By Boyden invasion chamber assay, we found that there was a significant decrease in the numbers of MDA-MB-231 and MDA-MB-435 cells transfected with the miR-10b inhibitor that traversed the matrigel-coated membrane compared with control groups. In addition, the miR-10b inhibitor could also inhibit the proliferation ability of MDA-MB-231 and MDA-MB-435 cells. These data suggested that miR-10b could take part in EMT in breast cancer and may be involved in invasion and metastasis through EMT.
The ability of TGF-β to induce EMT was initially described by Derynck and colleagues36 in 1994. During the intervening years since this important discovery, the findings of numerous studies have coalesced in establishing TGF-β as a master regulator of the initiation and resolution of EMT under a variety of pathophysiological contexts.29, 37 In order to examine whether miR-10b is involved in the TGF-β signaling pathway in EMT, we checked the miR-10b expression in MDA-MB-231 and MDA-MB-435 cells treated with TGF-β1 after 24 h and their corresponding parental cells. We found that, when stimulated with TGF-β1 for 24 h, MDA-MB-231 and MDA-MB-435 cells showed higher expression of miR-10b. Simultaneously, the expression of E-cadherin was decreased and the expression of vimentin was enhanced in MDA-MB-231 and MDA-MB-435 cells undergoing EMT. These studies led us to investigate whether miR-10b was a key factor for the TGF-β1-induced EMT in MDA-MB-231 and MDA-MB-435 cells. We found that the downregulation of miR-10b expression by miR-10b inhibitor treatment might partially reverse the E-cadherin and vimentin expression in MDA-MB-231 and MDA-MB-435 cells treated with TGF-β1 and that the miR-10b inhibitor could also rescue mesenchymal features of MDA-MB-231 and MDA-MB-435 cells, which were modulated by TGF-β1. Furthermore, the downregulation of miR-10b could fractionally restore the invasion and proliferation ability of MDA-MB-231 and MDA-MB-435 cells treated with TGF-β1. These data suggest that miR-10b might be a key factor in TGF-β1-induced EMT and might take part in the invasion and proliferation of breast cancer cells through EMT.
Taken together, our study demonstrates the contribution of miR-10b in TGF-β1-induced EMT, which might be one of the mechanisms that can be attributed to the metastatic function of miR-10b. A better understanding of the mechanisms of metastasis genes like miR-10b will provide greater insight into the metastasis of breast cancer, and miR-10b might be a potential therapeutic target for the treatment of metastasis of breast cancer.
References
Wu Q . Risks for breast cancer in Chinese female: a systematic review. Mod Prevent Med 2011; 38: 61–72.
Deakin NO, Turner CE . Distinct roles for paxillin and Hic-5 in regulating breast cancer cell morphology, invasion, and metastasis. Mol Biol Cell 2011; 22: 327–341.
Thiery JP, Sleeman JP . Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7: 131–142.
Yang J, Weinberg RA . Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008; 14: 818–829.
Nieto MA . The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 2011; 27: 347–376.
Gunaratne A, Thai BL, Di Guglielmo GM . Atypical protein kinase C phosphorylates Par6 and facilitates transforming growth factor β-induced epithelial-to-mesenchymal transition. Mol Cell Biol 2013; 33: 874–886.
Korc M, Friesel RE . The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets 2009; 9: 639–651.
Schönrath K, Klein-Szanto AJ, Braunewell KH . The putative tumor suppressor VILIP-1 counteracts epidermal growth factor-induced epidermal-mesenchymal transition in squamous carcinoma cells. PLoS ONE 2012; 7: e33116.
Ogunwobi OO, Liu C . Hepatocyte growth factor upregulation promotes carcinogenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via Akt and COX-2 pathways. Clin Exp Metastasis 2011; 28: 721–731.
Witsch E, Sela M, Yarden Y . Roles for growth factors in cancer progression. Physiology (Bethesda) 2010; 25: 85–101.
Kim MK, Maeng YI, Sung WJ, Oh HK, Park JB, Yoon GS et al. The differential expression of TGF-β1, ILK and wnt signaling inducing epithelial to mesenchymal transition in human renal fibrogenesis: an immunohistochemical study. Int J Clin Exp Pathol 2013; 6: 1747–1758.
Radisky ES, Radisky DC . Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia 2010; 15: 201–212.
Yan C, Grimm WA, Garner WL, Qin L, Travis T, Tan N et al. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-α through bone morphogenic protein-2. Am J Pathol 2010; 176: 2247–2258.
Wendt MK, Tian M, Schiemann WP . Deconstructing the mechanisms and consequences of TGF-β-induced EMT during cancer progression. Cell Tissue Res 2012; 347: 85–101.
Connolly EC, Freimuth J, Akhurst RJ . Akhurst. complexities of TGF-β targeted cancer therapy. Int J Biol Sci 2012; 8: 964–978.
Schetter AJ, Okayama H, Harris CC . The role of microRNAs in colorectal cancer. Cancer J 2012; 18: 244–252.
Iorio MV, Croce CM . MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med 2012; 4: 143–159.
Romero-Cordoba S, Rodriguez-Cuevas S, Rebollar-Vega R, Quintanar-Jurado V, Maffuz-Aziz A, Jimenez-Sanchez G et al. Identification and pathway analysis of microRNAs with no previous involvement in breast cancer. PLoS ONE 2012; 7: e31904.
Schetter AJ, Harris CC . Alterations of microRNAs contribute to colon carcinogenesis. Semin Oncol 2011; 38: 734–742.
Zhang ZJ, Ma SL . miRNAs in breast cancer tumorigenesis (Review). Oncol Rep 2012; 27: 903–910.
Ma L . Role of miR-10b in breast cancer metastasis. Breast Cancer Res 2010; 12: 210.
Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol 2010; 28: 341–347.
Li X, Xu F, Chang C, Byon J, Papayannopoulou T, Deeg HJ et al. Transcriptional regulation of miR-10a/b by TWIST-1 in myelodysplastic syndromes. Haematologica 2013; 98: 414–419.
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. . Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008; 68: 3645–3654.
Gupta M, Korol A, West-Mays JA . Nuclear translocation of myocardin-related transcription factor-A during transforming growth factor beta–induced epithelial to mesenchymal transition of lens epithelial cells. Mol Vis 2013; 19: 1017–1028.
Qian H, Yu J, Li Y et al. RNA interference of metastasis-associated gene 1 inhibits metastasis of B16F10 melanoma cells in a C57BL/6 mouse model. Biol Cell 2007; 99: 573–581.
Rao Y, Wang H, Fan L, Chen G. . Silencing MTA1 by RNAi reverses adhesion, migration and invasiveness of cervical cancer cells (SiHa) via altered expression of p53, and E-cadherin/β-catenin complex. J Huazhong Univ Sci Technolog Med Sci 2011; 31: 1–9.
Saito A, Suzuki HI, Horie M, Ohshima M, Morishita Y, Abiko Y et al. An integrated expression profiling reveals target genes of TGF-β and TNF-α possibly mediated by microRNAs in lung cancer cells. PLoS ONE 2013; 8: e56587.
Wendt MK, Allington TM, Schiemann WP. . Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol 2009; 5: 1145–1168.
Baum B, Settleman J, Quinlan MP . Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol 2008; 19: 294–308.
Micalizzi DS, Farabaugh SM, Ford HL . Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 2010; 15: 117–134.
Taylor MA, Parvani JG, Schiemann WP . The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-β in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia 2010; 15: 169–190.
Iorio MV, Croce CM . Causes and consequences of microRNA dysregulation. Cancer J 2012; 18: 215–222.
Liu Y, Zhao J, Zhang PY, Zhang Y, Sun SY, Yu SY et al. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med Sci Monit 2012; 18: BR299–BR308.
Misra A, Pandey C, Sze SK, Thanabalu T . Hypoxia activated EGFR signaling induces epithelial to mesenchymal transition (EMT). PLoS ONE 2012; 7: e49766.
Miettinen PJ, Ebner R, Lopez AR, Derynck R. . TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol 1994; 127 (6 Pt 2): 2021–2036.
Taylor MD, Liu Y, Nagji AS, Theodosakis N, Jones DR. . Combined proteasome and histone deacetylase inhibition attenuates epithelial-mesenchymal transition through E-cadherin in esophageal cancer cells. J Thorac Cardiovasc Surg 2010; 139: 1224–1232.
Acknowledgements
This work was supported by grants from the Medical Science and Technology Program of He’nan Province (13A320638).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Han, X., Yan, S., Weijie, Z. et al. Critical role of miR-10b in transforming growth factor-β1-induced epithelial–mesenchymal transition in breast cancer. Cancer Gene Ther 21, 60–67 (2014). https://doi.org/10.1038/cgt.2013.82
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2013.82
Keywords
This article is cited by
-
TGFβ signaling networks in ovarian cancer progression and plasticity
Clinical & Experimental Metastasis (2021)
-
A comprehensive review on oncogenic miRNAs in breast cancer
Journal of Genetics (2021)
-
Novel role of lncRNA CHRF in cisplatin resistance of ovarian cancer is mediated by miR-10b induced EMT and STAT3 signaling
Scientific Reports (2020)
-
miR-10b suppresses cell invasion and metastasis through targeting HOXA3 regulated by FAK/YAP signaling pathway in clear-cell renal cell carcinoma
BMC Nephrology (2019)
-
CADM2, as a new target of miR-10b, promotes tumor metastasis through FAK/AKT pathway in hepatocellular carcinoma
Journal of Experimental & Clinical Cancer Research (2018)