Abstract
Angiogenesis is required for normal physiologic processes, but it is also involved in tumor growth, progression and metastasis. Here, we report the development of an immune-based antiangiogenic strategy based on the generation of T lymphocytes that possess killing specificity for cells expressing vascular endothelial growth factor receptor 2 (VEGFR2). To target VEGFR2-expressing cells, we engineered cytotoxic T lymphocyte (CTL) expressing chimeric T-cell receptors (cTCR–CTL) comprised of a single-chain variable fragment (scFv) against VEGFR2 linked to an intracellular signaling sequence derived from the CD3ζ chain of the TCR and CD28 by retroviral gene transduction methods. The cTCR–CTL exhibited efficient killing specificity against VEGFR2 and a tumor-targeting function in vitro and in vivo. Reflecting such abilities, we confirmed that the cTCR–CTL strongly inhibited the growth of a variety of syngeneic tumors after adoptive transfer into tumor-bearing mice without consequent damage to normal tissue. In addition, CTL expressing both cTCR and tumor-specific TCR induced complete tumor regression due to enhanced tumor infiltration by the CTL and long-term antigen-specific function. These findings provide evidence that the tumor vessel-injuring ability improved the antitumor effect of CTLs in adoptive immunotherapy for a broad range of cancers by inducing immune-mediated destruction of the tumor neovasculature.
Similar content being viewed by others
Introduction
Adoptive immunotherapy, relying on the transfer of a large number of activated tumor-specific cytotoxic T lymphocytes (CTL), induces tumor regression in animal models and in human clinical trials.1, 2, 3 A major obstacle to clinical trials of adoptive immunotherapy, however, is represented by technical factors limiting the availability of adequate numbers of tumor-specific T cells to infuse.4 The specificity of activated CTL is mediated through the T-cell receptor (TCR) complex. Therefore, many researchers have suggested that pre-selected TCR gene transduction to CD8-positive T cells might be a valid tool to overcome such limitations, leading to the rapid generation of large amounts of tumor-specific T cells.5, 6, 7 In fact, data from the clinical trial of TCR gene transfer showed the feasibility of this approach in humans.7 Chimeric TCRs (cTCR), in which tumor antigen-specific recognition domains are combined with T-cell-activation domains in a single molecule, are also promising tools for cellular immunotherapy in cancer patients.8, 9, 10 These methods can be used to generate T cells with engineered specificities, thereby overcoming the lack of immunogenic tumor antigens and allowing for tumor cell recognition in a major histocompatibility complex-independent manner.8, 9, 10
Angiogenesis, the growth of new blood vessels from preexisting vessels, is a key contributor to tumor growth and metastasis due to the oxygen and nutrient supply provided.11 Because tumor-endothelial target structures are expressed in all solid tumors, targeting the established tumor vasculature may provide wide-ranging therapy. Novel approaches aim at targeting the tumor vasculature rather than the tumor cells.12, 13, 14 Vascular endothelial growth factor receptor 2 (VEGFR2), also known as fetal liver kinase 1 (flk1) in mouse and kinase insert domain-containing receptor in human, is a major receptor for crucial pro-angiogenic VEGF and is selectively expressed on endothelial cells and overexpressed on growing endothelial cells in tumor vasculature.15, 16, 17 Because angiogenesis is indispensable for the growth of numerous tumors, flk1 is a candidate target molecule for anticancer drugs.18
In the present study, we generated gene-modified CTL to target flk1-expressing cells as tumor-endothelial cells, and evaluated their antitumor efficacy and broad utility in adoptive immunotherapy. We previously demonstrated, using a retroviral vector system, that the transfer of CTL expressing an anti-flk1 single-chain variable fragment (scFv; scFv–CTL) enhanced tumor infiltration.19 We subsequently generated CTL expressing an anti-flk1 cTCR that contained anti-flk1 scFv as the antigen recognition motif and the cytoplasmic region of CD3ζ chains and CD28 as the T-cell activation motif, which we named cTCR–CTL. Moreover, we assumed that CTL expressing both anti-flk1 cTCR and tumor antigen-specific TCR (CTL expressing dual TCR, which we named dTCR–CTL) would be directly accessible to tumor cells and could exert an even more powerful antitumor effect, because the tumor vessel-injuring ability would facilitate the extravasation of CTL from the bloodstream to the tumor tissue. Here, we demonstrate the tumor vessel-injuring ability of cTCR–CTL or dTCR–CTL in vitro and in vivo, and report the antitumor activity and immune response after adoptive transfer.
Materials and methods
Cell lines and mice
Murine islet endothelial MS1 cells (H-2b) and E.G7-OVA cells (H-2b) were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and in RPMI 1640 medium supplemented with 10% FBS, 50 μM 2-melcaptoethanol and 400 μg ml−1 G418, respectively. The murine melanoma B16BL6 cells (H-2b) were obtained from the JCRB cell bank (Tokyo, Japan) and cultured in minimum essential medium supplemented with 7.5% FBS. Lewis lung carcinoma 3LL cells (H-2b) were purchased from RIKEN BioResource Center (Ibaraki, Japan) and cultured in DMEM supplemented with 10% FBS. Murine fibrosarcoma Meth-A cells (H-2d) were kindly provided by Dr H Fujiwara and maintained by intraperitoneal passage in syngeneic BALB/c mice. Murine colon carcinoma CT26 cells (H-2d) were kindly provided by Prof NP Restifo (National Cancer Institute, Bethesda, MD, USA) and grown in RPMI 1640 medium supplemented with 10% FBS. PLAT-E cells,20 a helper cell line for retrovirus propagation, were kindly provided by Prof T Kitamura (Tokyo University, Tokyo, Japan) and cultured in DMEM supplemented with 10% FBS, 1 μg ml−1 puromycin and 10 μg ml−1 brasticidin.
Female C57BL/6 mice (H-2b) and BALB/c mice (H-2d) were purchased from Japan SLC Inc. (Hamamatsu, Japan). Pmel-1 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Animal experimental procedures were performed in accordance with the Osaka University guidelines for the welfare of animals in experimental neoplasia.
Construction of retroviral vector expressing anti-flk1 cTCR
An anti-flk1 scFv gene was previously generated from cDNA extracted from Avas12α1 hybridoma cells,19, 21 which were kindly provided by Prof S Nishikawa (RIKEN, Kobe, Japan). Anti-flk1 cTCR contains the anti-flk1 scFv and cytoplasmic region of CD3ζ and CD28. The gene for the cytoplasmic CD3ζ or CD28 region was amplified from the mouse spleen cDNA library (Agilent Technologies Inc., Santa Clara, CA, USA) by PCR (94 °C × 1 min, 60 °C × 45 s and 72 °C × 1 min; 35 cycles) using their respective specific primers (CD3ζ-region: forward 5′-CAGAGACTTTGCAGCGTACCGCCCCAGAGCAAAATTCAGCAGGAGTGCAG-3′, including a part of the CD28 sequence; reverse 5′-GCAGCGCGGCCGCTTAGCGAGGGGCCAGGG-3′, including NotI site; CD28 region: forward 5′-CGGGACTTTCCAAAATGCCGCGGATTGAGTTCATGTACCCTCCGCCTTAC-3′, including SacII site; reverse 5′-GGGGCGGTACGCTGCAAAG-3′, a part of the CD3ζ sequence). The cDNAs of the CD3ζ and CD28 were assembled by three cycles of PCR (94 °C × 1 min, 63 °C × 30 s, 58 °C × 50 s, and 72 °C × 1 min) using a DNA linker fragment. The assembled scFv fragment was reamplified using both CD3ζ reverse and CD28 forward primers. The resulting fragment was digested with SacII and NotI and ligated into the pMXs-IG vector containing cDNA of anti-flk1 scFv,19 a retroviral plasmid carrying EGFP and anti-flk1 scFv. The PLAT-E cells were transfected with these expression vectors using FuGENE 6 (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s recommendations. The medium was changed 1 day after transfection, and retroviral vectors were collected 48 h after transfection, as previously described.19, 22
Gene transfer to CTL
The CD8-positive T lymphocytes were purified from murine splenocytes and lymph node cells by using a CD8-isolation kit and Auto MACS (Miltenyi Biotec, Auburn, CA, USA) according to manufacturer’s specifications. CD8-positive T lymphocytes were activated by anti-CD3 mAb (clone 145-2C11, eBioscience, San Diego, CA, USA) and costimulated with anti-CD28 mAb (clone 37.51, eBioscience) for 72 h in RPMI 1640 supplemented with 10% FBS, 50 μM 2-melcaptoethanol and 10 U ml−1 IL-2 (PeproTech Inc., Rocky Hill, NJ, USA). The viral supernatant was loaded onto plates coated with recombinant fibronectin fragment CH-296, RetroNectin (TaKaRa Bio Inc., Ohtsu, Japan) according to the manufacturer’s instructions, and incubated for 4 h. The virus-coating procedure was repeated twice. Before infection, the viral supernatant was washed away and the activated CD8-positive CTL were added on the virus-coating plate. CTL were cultured for 48 h to allow infection to occur. Gene-transduced CTL were sorted by FACSAria (BD Biosciences, San Jose, CA, USA), and cultured for amplification in complete medium with IL-2 (10 U ml−1) for 3 days.
Flow cytometric analysis for anti-flk1 scFv or anti-flk1 cTCR expression
Gene-transduced CTL (scFv–CTL or cTCR–CTL) were incubated with 100 μl staining buffer (phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin and 0.01% NaN3) containing recombinant mouse flk1 Fc chimera (R&D Systems Inc., Minneapolis, MN, USA) labeled with Zenon technology with R-phycoerythrin Human IgG-labeling reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After incubation for 30 min, the cells were washed with staining buffer, and then 30 000 events of the stained cells were acquired on a FACSCalibur flow cytometer (BD Biosciences), and analyzed for anti-flk1 scFv or anti-flk1 cTCR protein expression using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Evaluation of cytotoxic activity
Cytotoxic specificity was determined in standard 51Cr-release assays. Target cells (MS1 and B16BL6) were 51Cr-labeled and incubated with gene-transduced CTL or mock CTL for 4 h at 37 °C. Cytolytic activity was determined based on the following formula: (% of lysis)=((experimental 51Cr release−spontaneous 51Cr release)/ (maximum 51Cr release−spontaneous 51Cr release)) × 100. The spontaneous 51Cr release of the target cells was <10% of the maximum 51Cr release induced by detergent.
Evaluation of tumor growth
In the solid tumor model, C57BL/6 mice (H-2b) were intradermally inoculated with 2 × 105 B16BL6 cells (H-2b) or 3 × 105 3LL cells (H-2b) into the right flank, and BALB/c mice (H-2d) were intradermally inoculated with 5 × 105 Meth-A cells (H-2d) or 4 × 105 CT26 cells (H-2d) into the right flank. Seven days later, mice bearing tumors with diameters of 5.5–6.5 mm were intravenous (i.v.) injected with CTL in 100 μl PBS. Tumor growth was monitored two or three times a week by measuring the major and minor axes of the tumors using microcalipers, and tumor volume was calculated according to the following formula: (tumor volume; mm3)=(major axis; mm) × (minor axis; mm)2 × 0.5236. The mice were euthanized when one of the two measurements was >20 mm. In the metastasis model, C57BL/6 mice were i.v. injected with 3 × 105 B16BL6 cells. Eight days later, mice were i.v. injected with indicated CTL in 100 μl PBS. Six days after CTL injection, the lungs were collected from these mice, and the numbers of metastatic nodules were counted.
Wound-healing assay
Full-thickness skin circular wounds of 6 mm diameter were surgically created on the backs of C57BL/6 mice. After creating the wounds, mice were treated with 5 × 106 cTCR–CTL, scFv–CTL or PBS at 2, 4 and 6 days. The wound area was monitored two or three times a week by measuring the major and minor axes of the wound using microcalipers, and the wound area was calculated according to the following formula: (wound area; mm2)=(major axis; mm) × (minor axis; mm) × 3.14.
Histopathologic examination of kidney sections
C57BL/6 mice bearing B16BL6 were i.v. injected with CTL in 100 μl PBS. Eight days after CTL injection, the kidneys were collected from these mice, placed in neutral 10% formalin/PBS and embedded in paraffin. Sections (5 μm thick) were prepared for hematoxylin and eosin staining, and then histopathologic examination was performed at the Applied Medical Research Laboratory (Osaka, Japan).
Accumulation of transferred CTL in tumor tissues
C57BL/6 mice were intradermally inoculated with 2 × 105 B16BL6 cells into the right flank. Seven days later, mice bearing tumors with diameters of 5.5–6.5 mm were i.v. injected with 2.5 × 106 CTLs labeled with PKH26 dyes in 100 μl PBS. The tumors and regional lymph nodes were dissected out on the indicated days after CTL transfer and chopped into small pieces using a razor blade before incubation with a mixture of collagenase (1 mg ml−1, Wako Pure Chemical Industries, Ltd., Osaka, Japan) dissolved in Hanks’ balanced salt solution for 60 min at 37 °C. The cells were passed through a 70 μm nylon strainer to remove any debris, recovered by centrifugation and resuspended in complete medium. The frequency of PKH26-positive CTL was assessed by flow cytometric analysis acquiring 100 000 events. The number of CTL that accumulated in the tumor was calculated by multiplying the number of PKH26-positive cells by the total number of isolated tumor cells.
Evaluation of CTL activity in gene-transduced CTL-injected mice
CD8-positive T cells were isolated from the spleen and regional lymph nodes of tumor-regressed mice, and then restimulated for 4 days in culture medium with gp10025–33 peptide-pulsed dendritic cells. Cytotoxic specificity was determined in standard 51Cr-release assays. Target cells (MS1, B16BL6, E.G7-OVA) were 51Cr-labeled and incubated with stimulated CTL for 4 h at 37°C. Cytolytic activity was determined based on the following formula: (% of lysis)=((experimental 51Cr release−spontaneous 51Cr release)/ (maximum 51Cr release−spontaneous 51Cr release)) × 100. The spontaneous 51Cr release of the target cells was <10% of the maximum 51Cr release induced by detergent.
Results
Analysis of gene-transduced CTL expressing anti-flk1 cTCR
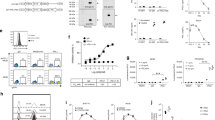
We achieved the genetic modification of primary T cells with anti-flk1 cTCR using a retroviral-based gene transduction method. First, we assessed the expression levels of anti-flk1 scFv on cTCR–CTL by flow cytometry analysis. As shown in Figure 1a, cTCR–CTL bound to more flk1/Fc chimera equal with scFv–CTL as compared with CTL treated with control vector (sham CTL). This result indicated that anti-flk1 scFv on CTL recognized native flk1 on the cell surface, and that anti-flk1 cTCR gene-transduced CTL had tumor vessel–targeting activity similar to scFv–CTL. We next used a standard 51Cr-release assay to investigate whether cTCR–CTL could kill MS1 cells expressing flk1 molecules. cTCR–CTL derived from both C57BL/6 and BALB/c mice exhibited high cytotoxic activity against MS1 cells, whereas B16BL6 cells expressing no flk1 were not injured (Figure 1b). Ligand binding by the anti-flk1 scFv domain of the cTCR triggered phosphorylation of the immunoreceptor tyrosine-based activation motif in the cytoplasmic region of the molecules (data not shown), which activated a signaling cascade that was required for the induction of cytolysis. Antigen recognition was therefore not major histocompatibility complex-restricted, but instead was directed to the cell-surface flk1 molecules.
Cytotoxic activity of CTL expressing anti-flk1 cTCR. CTL were purified from C57BL/6 or BALB/c mice and transduced with anti-flk1 cTCR or anti-flk1 scFv-expressing retroviral vectors. (a) Forty-eight hours after gene transduction, the expression level of anti-flk1 scFv in CTL was analyzed by flow cytometry. Filled histogram represents the control; the dashed line, scFv–CTL; solid line, cTCR–CTL. (b) Anti-flk1 cTCR or anti-flk1 scFv-expressing CTL (cTCR–CTL or scFv–CTL) as effector cells were co-cultured with MS1 cells or B16BL6 cells as target cells at varying effector-to-target cell ratios. Four hours later, the cytotoxic activity of cTCR–CTL or scFv–CTL was determined using a standard 51Cr-release assay. Each point represents the mean±s.d. of three independent cultures.
Taken together, these findings demonstrate that anti-flk1 cTCR expressed on CTL possessed sufficient ability to recognize the flk1 molecules on the vascular endothelial cells, and that the intracellular domain of anti-flk1 cTCR led to an flk1-specific cytotoxic signal through binding of the anti-flk1 scFv to the flk1 molecule.
Antitumor effect of gene-transduced CTL expressing anti-flk1 cTCR
To evaluate the antitumor effects of gene-modified CTL with tumor vessel-injuring ability and few tumor-specific TCR, we monitored tumor growth in mice systemically injected with cTCR–CTL or scFv–CTL in various tumor models. As shown in Figure 2a, tumor growth in mice transferred with scFv–CTLs was nearly equal to that of tumors in mice transferred with PBS (control). In contrast, mice transferred with cTCR–CTL exhibited an obvious growth delay as compared with scFv–CTL transfer groups in all tested tumor models. Moreover, in a melanoma metastasis model, there were remarkably fewer metastatic nodules in mice treated with cTCR–CTL than in mice treated with non-transduced CTL or scFv–CTL as the control, in a dose-dependent manner (Figure 2b). In addition, scFv–CTL showed a slight antitumor effect despite the lack of tumor-specific TCR. Activated CTL can injure their target predominantly via the granule exocytosis pathway involving perforin, and also mediate apoptotic activity through FasL and the TNF-related apoptosis-inducing ligand pathway.23, 24 Therefore, the antitumor effect of scFv–CTL transfer is considered to efficiently induce apoptosis through FasL, based on the accumulation of their activity in tumor tissue in vivo.
Antitumor efficacy of CTL expressing anti-flk1 cTCR. CTL were purified from C57BL/6 or BALB/c mice and transduced with anti-flk1 cTCR or anti-flk1 scFv-expressing retroviral vectors. (a) C57BL6 mice bearing B16BL6 or 3LL and BALB/c mice bearing Meth-A, or CT26 tumors were i.v. injected with 5 × 106 anti-flk1 cTCR or anti-flk1 scFv-expressing CTL (cTCR–CTL or scFv–CTL). As a control, PBS was i.v. injected into tumor-bearing mice. The tumor volume was calculated after measuring the major and minor axes of the tumor at indicated points. Each point represents the mean±s.d. from six mice. (b) C57BL/6 mice were i.v. injected with 3 × 105 B16BL6 cells (day 0). Eight days later, 5 × 106 or 1 × 106 CTL were i.v. injected. At 14 days after metastasis, the lungs were collected from these mice, and the number of metastatic nodules was counted. Data are presented as means±s.d. from six mice. Statistical analysis was performed using one-way analysis of variance: *P<0.01 versus the scFv–CTL (1 × 106)-injected group; † P<0.01 versus the scFv–CTL (5 × 106)-injected group; ‡ P<0.01 versus the cTCR–CTL (1 × 106)-injected group.
Expression of anti-flk1 cTCR in CTL was effective in various solid tumor and metastasis models without tumor-specific TCR via tumor vessel-injuring ability because tumor vascular endothelial cells are common to all solid tumor tissues.
Antiangiogenic activity of gene-transduced CTL expressing anti-flk1 cTCR
We then investigated whether the antitumor effect of cTCR–CTL was involved in antiangiogenesis. Angiogenesis is involved in a variety of pathologic responses, including tumor growth, metastasis and chronic inflammation.11, 25 Moreover, the wound-healing process is highly dependent on angiogenesis.26, 27 Therefore, we performed wound-healing assays as described in the Materials and Methods section to determine the effect of cTCR–CTL on angiogenesis in vivo. As shown in Figure 3a, there were significant differences between the wound areas of the cTCR–CTL-treated group and the scFv–CTL-treated group at 9 days after wounding. scFv–CTL-treated mice and PBS-treated mice showed earlier healing, and some wounds were completely healed within 15 days after wounding (Figure 3b). In contrast, cTCR–CTL treatment suppressed wound healing in mice and 20 days was required for all wounds to heal. These findings indicate that treatment with cTCR–CTL significantly delayed wound healing as a result of the inhibition of angiogenesis, and thus the antitumor effect of cTCR–CTL is attributed to cytotoxic activity against flk1-expressing tumor vessel endothelial cells. In addition, we confirmed whether cTCR–CTL treatment caused toxicity, which is associated with angiogenesis inhibition. The kidney highly expresses flk1 molecules and is highly vascular.28 Therefore, we evaluated the kidneys of B16BL6-bearing mice treated with cTCR–CTL. Figure 4 shows that the kidneys in mice transferred with scFv–CTL showed no histopathologic changes. Moreover, cTCR–CTL did not damage the kidneys, and the toxicity score was 0 (no) in all treatment groups. It was assumed that the flk1 expression level is lower in normal tissue than in tumor tissue. Therefore, these results indicate that treatment with cTCR–CTL had little effect on normal tissue.
Effect of cTCR–CTL injection on wound healing. A circular wound of 6 mm diameter was created on the upper back of C57BL/6 mice. After creating the wound, 5 × 106 cTCR–CTL, 5 × 106 scFv–CTL (C57BL/6) or PBS was i.v. injected on each of the days 2, 4 and 6 into C57BL/6 mice. (a) The neovascularity-injuring activity was analyzed based on morphology at day 9. (b) The wound area was calculated after measuring the major and minor axes of the wound at the indicated points. Each point represents the mean±s.d. from six mice. Statistical analysis was performed using one-way analysis of variance: *P<0.01 versus scFv–CTL-injected group.
Antitumor effect of CTL expressing both anti-flk1 cTCR and tumor-specific TCR
We selected the CD8-positive T cells derived from pmel-1 transgenic mice, whose CD8-positive T cells recognize the gp10025-33 (KVPRNQDWL) peptide in the context of H-2b on a C57BL/6 background, as a model of tumor-specific TCR gene-transferred CTL. First, to confirm whether the two antigen receptors (anti-flk1 cTCR and gp100-specific TCR) in dTCR–CTL maintained their conventional functions, we performed a cytolytic assay using dTCR–CTL prepared from CTL derived from pmel-1 mice against flk1-expressing cells and gp100. Figure 5 shows that non-transduced CTL and scFv–CTL derived from pmel-1 mice kill B16BL6 melanoma cells expressing gp100, but not both MS1 cells and E.G7-OVA cells because of the scarce expression of gp100 molecules. This finding indicates that anti-flk1 scFv expressed on CTL did not affect original cytolytic activity of CTL. Further, dTCR–CTL as well as cTCR–CTL exhibited high cytotoxic activity against MS1 cells. In addition, dTCR–CTL, but not cTCR–CTL, killed B16BL6. Therefore, these results indicate that each antigen receptor on dTCR–CTL maintained its own antigen-specific cytotoxic activities.
Flk1 and gp100-specific cytotoxic activities of dTCR–CTL. CTL were purified from pmel-1 or C57BL/6 mice and transduced with anti-flk1 cTCR or anti-flk1 scFv-expressing retroviral vectors. Cytotoxic activities of each CTL were evaluated by 51Cr-release assay using MS1 cells, B16BL6 cells, and E.G7-OVA cells as target cells. Each point represents the mean±s.d., and of three independent cultures.
To compare the antitumor effect of dTCR–CTL with that of scFv–CTL derived from pmel-1 mice or that of cTCR–CTL derived from C57BL/6 mice in melanoma models, we investigated changes in the tumor size and survival ratios of mice tested after adoptive transfer with various CTL. As shown in Figure 6a, adoptive transfer of both scFv–CTL (pmel-1; 106 cells) and cTCR–CTL (C57BL/6; 5 × 106 cells) to B16BL6-bearing mice delayed tumor growth compared with that of PBS (control group) and non-transduced CTL (pmel-1; 106 cells), and resulted in reoccurrence in each group. On the other hand, adoptive transfer of dTCR–CTL (pmel-1; 106 cells) induced obvious suppressive effects on B16BL6 tumor growth regardless of the dose, and even achieved complete regression in three of seven mice (Figure 6b).
Antitumor efficacy of dTCR–CTL. CTL were purified from pmel-1 or C57BL/6 mice and transduced with anti-flk1 cTCR or anti-flk1 scFv-expressing retroviral vectors. Non-transduced CTL, scFv–CTL and dTCR–CTL derived from pmel-1 mice (106 cells) and cTCR–CTL derived from C57BL/6 mice (5 × 106 cells) were i.v. injected into B16BL6 tumor-bearing mice. As a control, PBS was i.v.-injected into some tumor-bearing mice. (a) The tumor volume was calculated after measuring the major and minor axes of the tumor at indicated points. Each point represents the mean±s.d. from seven mice. (b) Data represents the number of mice for which tumors were <20 mm, expressed as a percentage of the total mice tested in each group.
These findings indicate that the approach to target either tumor cells or tumor vessel endothelial cells was insufficient to induce complete tumor regression. Moreover, our strategy is suitable for adoptive immunotherapy against solid tumor tissues, because tumor-specific TCR easily attacked tumor cells after the destruction of tumor vascular endothelial cells.
Long-term effect of CTL expressing both anti-flk1 cTCR and tumor-specific TCR
To analyze the differentiation of CTL to the memory phase in vivo after transfer, we first estimated the number of CTL that accumulated in the tumor tissues or regional lymph nodes. Three days after CTL transfer, the number of tumor-accumulated cTCR–CTL and dTCR–CTL was greater than that of control CTL and equal to that of scFv–CTL in both tumor tissues and regional lymph nodes (Figure 7a). In addition, the number of cTCR–CTL and dTCR–CTL detected in tumors and lymph nodes on days 7 and 11 was increased compared with that on day 3. Expression of anti-flk1 scFv only enhanced CTL accumulation, because the number of scFv–CTL detected in tumors on day 7 or day 11 was equal to that on day 3. These findings indicate that cTCR–CTL or dTCR–CTL proliferated because of recognition of the flk1 molecule through its own cTCR.
Long-term functionality of cTCR–CTL and dTCR–CTL in vivo. CTL were purified from pmel-1 or C57BL/6 mice and transduced with anti-flk1 cTCR or anti-flk1 scFv-expressing retroviral vectors. Each CTL was i.v. injected into B16BL6 tumor-bearing mice. As a control, PBS was i.v. injected into some tumor-bearing mice. (a) CTL (2.5 × 106 cells) labeled with PKH26 dyes were i.v. injected into B16BL6 tumor-bearing C57BL/6 mice. The tumor and lymph nodes were removed for preparation of a cell suspension for flow cytometric analysis on day 3 (open square), day 7 (dotted square), day 11 (hatched square) or day 21 (filled square) after CTL injection. Data are presented as mean±s.d. of results from three mice. (b) Three months after injection of 5 × 106 dTCR–CTLs into C57BL/6 mice bearing well-established (∼6 to 6.5 mm in diameter) B16BL6 tumors, CD8-positive T cells were prepared from mice that achieved complete regression of the primary tumor, and then were restimulated with gp10025-33-pulsed dendritic cells for 4 days. The flk1 and gp100-specific cytotoxic activities of these CTL (•) or CTL isolated from C57BL6 (♦) were evaluated by a 51Cr-release assay using MS1 cells, B16BL6 cells, and E.G7-OVA as target cells. Each point represents the mean±s.d. of three independent cultures.
Moreover, we discovered that the splenocytes in mice achieving complete regression of primary B16BL6 tumors by treatment with dTCR–CTL exhibited high cytotoxic activity against MS1 cells and B16BL6 in an effector-to-target cell ratio-dependent manner, whereas E.G7-OVA cells were not injured (Figure 7b). This finding indicates that mice adoptively transferred with dTCR–CTL had lymphocytes possessing the ability to kill both flk1 and gp100-expressing cells, and that dTCR–CTL had long-term effects. It is expected that immunologic self-tolerance establishes in flk1 molecules in tumor-bearing mice. Therefore, these data indicate that treatment with dTCR–CTL led to the establishment of memory T cells against flk1 molecules through the cTCR signal.
Discussion
CTL can injure tumor cells upon recognizing antigenic peptides presented via major histocompatibility complex molecules on tumor cells through their tumor-specific TCR.29 Cancer immunotherapy approaches are aimed at activating CTL as the major effector cells in antitumor immune responses. Adoptive T-cell therapy, which involves the ex vivo selection and expansion of antigen-specific CTL, augments antigen-specific immunity without the in vivo constraints associated with vaccine-based strategies. In particular, the TCR gene transfer technique allows for the generation of T cells with a defined tumor-associated antigen specificity, even when this specificity is lacking in the endogenous T-cell repertoire.30 To eradicate tumors, however, CTL must act in the microenvironment that has been established by the tumor. This environment typically promotes angiogenesis through the expression of pro-angiogenic cytokines, for example, VEGF, and limits the extravasation of lymphocytes from tumor vessels into the tumor stroma to enhance tumor growth. The therapeutic effect of neutralizing monoclonal anti-VEGF or anti-VEGFR2 antibodies alone has been reported.31, 32, 33 The antiangiogenic agents used in these previous studies, however, had minimal clinical impact when used alone. On the other hand, these studies demonstrated a clinical effect when administered in conjugation with chemotherapy and adoptive immunotherapy.33, 34, 35, 36 A recent report demonstrated that inhibition of the VEGF/VEGFR2 axis increases the extravasation of adoptively transferred T cells into the tumor.36 Therefore, it is important to disrupt both tumor vessels and the parenchyma. We previously demonstrated, using the anti-flk1 scFv expression method, that tumor vessel-targeting CTL efficiently accumulate in the tumor tissue to induce a more powerful antitumor effect.19 Here, we showed that gene-modified CTL with both tumor-targeting and tumor vessel-injuring abilities enhance infiltration into the tumor site to improve the antitumor effect of adoptive immunotherapy.
In the present study, we first showed that anti-flk1 cTCR mimics the TCR-specific flk1 molecules, which are overexpressed on tumor vascular endothelial cells. Activation of naïve T cells requires two signals from mature antigen-presenting cells.37 Our cTCR–CTL expressed a chimeric receptor containing anti-flk1 scFv and the intracellular domain of not only the CD3ζ chain but also CD28. This construct initiates costimulatory signaling to the nucleus after antigen engagement, and thereby helps to compensate for the lack of physiologic costimulation when cTCR engage target cells without anergy, for example, proliferative capacity and IL-2 secretion.38, 39 The expression of anti-flk1 cTCR on CTL contributed to the binding capacity of flk1-expressing cells in vitro and the accumulation into tumor tissue in vivo as well as anti-flk1 scFv on CTL. Moreover, cTCR–CTL exerted the antitumor effect on various tumor types through the destruction of flk1-expressing cells in the tumor tissue rather than through their direct cytotoxicity against tumor cell itself. This tumor vessel-targeting approach provides a potential method for a variety of human cancers because tumor-specific molecules and their major histocompatibility complex-restricted TCR identification are unnecessary. The utility of our strategy, expressing anti-flk1 cTCR on naïve CD8-positive T cells, is supported by the findings of another research group.40 There is some concern, however, about the damage to normal tissue in targeting angiogenesis or the recognition of a low level of flk1. In the present study, we showed that cTCR–CTL did not damage the kidney, but did delay wound healing. It is important to consider normal angiogenesis in the protocol of adoptive immunotherapy using antiangiogenesis CTL. We also demonstrated that CTL expressing both anti-flk1 cTCR and tumor-specific TCR exhibited more powerful antitumor effects than CTL expressing anti-flk1 cTCR alone or tumor-specific TCR alone. These results indicate a need for the destruction of both tumor vessel endothelial cells and tumor parenchymal cells.
The in vitro activation of T cells that is required for retroviral gene transfer leads to phenotypic maturation, including the downregulation of CD62L expression. As a consequence, gene-modified T cells may have a reduced capacity for lymph node entry, thereby reducing antigen responsiveness shortly after infusion.41 In the present study, however, we showed that cTCR–CTL and dTCR–CTL are both present in the tumor tissue and lymph nodes in a time-dependent manner. These findings raise the possibility that infused cTCR–CTL and dTCR–CTL differentiated into memory T cells, which re-expressed CD62L. This is supported by our data that dTCR–CTL survived for 3 months after adoptive transfer and maintained both their ability to react to a secondary antigen encounter in vivo and their long-term antigen-specific cytotoxic functionality. The CD80/CD86–CD28 costimulatory pathway is the major costimulatory pathway and it is critical for primary CD8-positive T-cell responses.42 Recent studies using CD28-knockout mice demonstrated that CD28 costimulation not only controls the magnitude of the primary response but also affects the development of memory CD8-positive T cells, and is required during the recall response in addition to initial T-cell priming.43 Therefore, the cytoplasmic region of CD28 in cTCR molecules may contribute to differentiation into memory T cells for each ligand-binding signal by the anti-flk1 scFv domain. Although T-cell activation leads to the upregulation of CTLA-4, which binds to CD80 and CD86 at a higher affinity than CD28 and negatively regulates the T-cell response,44 activated cTCR–CTL delivered a CD28-associated signal without the need for CD80/CD86 binding.
Conclusion
The findings of the present study demonstrate that the expression of anti-flk1 cTCR on tumor-specific CTL resulted in a powerful antitumor effect due to both their accumulation in tumor tissue and their killing function against tumor cells in one cell. In addition, our findings suggest that dTCR–CTL greatly contribute to differentiation into memory cells. Generally, tumor-specific CTL are expanded from tumor-infiltrating cells or peripheral blood mononuclear cells by stimulation with a tumor antigen peptide plus IL-2 or dissociated tumor lesions plus IL-2.3 It is difficult, however, to obtain large numbers of antigen-specific CTL from tumor-infiltrating cells or peripheral blood mononuclear cells. Therefore, our strategy to add the injury function against flk1-expressing cells to tumor-specific CTL will be useful for adoptive immunotherapy due to their tumor specificity because fewer CTL are necessary. Moreover, the combination of tumor-specific TCR and anti-flk1 cTCR expression methods may improve the availability of lymphocytes derived from cancer patients. Our technology and methodology also will likely have far-reaching implications for hundreds of other types of cell therapies.
References
Gattinoni L, Powell DJ, Rosenberg SA, Restifo NP . Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 2006; 6: 383–393.
Fang L, Lonsdorf AS, Hwang ST . Immunotherapy for advanced melanoma. J Invest Dermatol 2008; 128: 2596–2605.
Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl. Cancer Inst 1994; 86: 1159–1166.
Dudley ME, Rosenberg SA . Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 2003; 3: 666–675.
Stauss HJ, Cesco-Gaspere M, Thomas S, Hart DP, Xue SA, Holler A et al. Monoclonal T-cell receptors: new reagents for cancer therapy. Mol Ther 2007; 15: 1744–1750.
de Witte MA, Jorritsma A, Kaiser A, van den Boom MD, Dokter M, Bendle GM et al. Requirements for effective antitumor responses of TCR transduced T cells. J Immunol 2008; 181: 5128–5136.
Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006; 314: 126–129.
Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE . The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J Immunol 2010; 184: 6938–6949.
Altvater B, Landmeier S, Pscherer S, Temme J, Juergens H, Pule M et al. 2B4 (CD244) signaling via chimeric receptors costimulates tumor-antigen specific proliferation and in vitro expansion of human T cells. Cancer Immunol Immunother 2009; 58: 1991–2001.
Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M . Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther 2010; 18: 413–420.
Kerbel RS . Tumor angiogenesis. N Engl J Med 2008; 358: 2039–2049.
Cho SG, Yi Z, Pang X, Yi T, Wang Y, Luo J et al. Kisspeptin-10, a KISS1-derived decapeptide, inhibits tumor angiogenesis by suppressing Sp1-mediated VEGF expression and FAK/Rho GTPase activation. Cancer Res 2009; 69: 7062–7070.
Grothey A, Galanis E . Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol 2009; 6: 507–518.
Kiselyov A, Balakin KV, Tkachenko SE . VEGF/VEGFR signalling as a target for inhibiting angiogenesis. Expert Opin Investig Drugs 2007; 16: 83–107.
Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Dvorak HF et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol 1993; 143: 1255–1262.
Plate KH, Breier G, Millauer B, Ullrich A, Risau W . Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res 1993; 53: 5822–5827.
Brown LF, Olbricht SM, Berse B, Jackman RW, Matsueda G, Tognazzi KA et al. Overexpression of vascular permeability factor (VPF/VEGF) and its endothelial cell receptors in delayed hypersensitivity skin reactions. J Immunol 1995; 154: 2801–2807.
Ramakrishnan S, Olson TA, Bautch VL, Mohanraj D . Vascular endothelial growth factor-toxin conjugate specifically inhibits KDR/flk-1-positive endothelial cell proliferation in vitro and angiogenesis in vivo. Cancer Res 1996; 56: 1324–1330.
Kanagawa N, Yanagawa T, Mukai Y, Yoshioka Y, Okada N, Nakagawa S et al. Tumor-targeting CTL expressing a single-chain Fv specific for VEGFR2. Biochem Biophys Res Commun 2010; 394: 54–58.
Morita S, Kojima T, Kitamura T . Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther 2000; 7: 1063–1066.
Kataoka H, Takakura N, Nishikawa S, Tsuchida K, Kodama H, Kunisada T et al. Expressions of PDGF receptor alpha, c-Kit and Flk1 genes clustering in mouse chromosome 5 define distinct subsets of nascent mesodermal cells. Dev Growth Differ 1997; 39: 729–740.
Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol 2003; 31: 1007–1014.
Kreuwel HT, Morgan DJ, Krahl T, Ko A, Sarvetnick N, Sherman LA . Comparing the relative role of perforin/granzyme versus Fas/Fas ligand cytotoxic pathways in CD8+ T cell-mediated insulin-dependent diabetes mellitus. J Immunol 1999; 163: 4335–4341.
Dace DS, Chen PW, Niederkorn JY . CD8+ T cells circumvent immune privilege in the eye and mediate intraocular tumor rejection by a TNF-alpha-dependent mechanism. J Immunol 2007; 178: 6115–6122.
Griffioen AW, Molema G . Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev 2000; 53: 237–268.
Risau W . Mechanisms of angiogenesis. Nature 1997; 386: 671–674.
Zhang N, Fang Z, Contag PR, Purchio AF, West DB . Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood 2004; 103: 617–626.
Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med 2006; 47: 2048–2056.
Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P . Human T cell responses against melanoma. Annu Rev Immunol 2006; 24: 175–208.
Sadelain M, Rivière I, Brentjens R . Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer 2003; 3: 35–45.
Prewett M, Huber J, Li Y, Santiago A, O’Connor W, King K et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res 1999; 59: 5209–5218.
Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010; 28: 780–787.
Jain RK, Duda DG, Clark JW, Loeffler JS . Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol 2010; 3: 24–40.
Crawford Y, Ferrara N . VEGF inhibition: insights from preclinical and clinical studies. Cell Tissue Res 2009; 335: 261–269.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–2342.
Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA . Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010; 70: 6171–6180.
Acuto O, Michel F . CD28-mediated co-stimulation: a quantitative support for TCR signaling. Nat Rev Immunol 2003; 3: 939–951.
Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol 2010; 167: 6123–6131.
Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M . Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol 2005; 20: 70–75.
Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest 2010; 120: 3953–3968.
Xu J, Grewal IS, Geba GP, Flavell RA . Impaired primary T cell responses in L-selectin-deficient mice. J Exp Med 1996; 183: 589–598.
Kündig TM, Shahinian A, Kawai K, Mittrücker HW, Sebzda E, Bachmann MF et al. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity 1996; 5: 41–52.
Fuse S, Zhang W, Usherwood EJ . Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J Immunol 2008; 180: 1148–1157.
Egen JG, Kuhns MS, Allison JP . CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002; 3: 611–618.
Acknowledgements
We thank Professor Toshio Kitamura (Division of Cellular Therapy, Advanced Clinical Research Center, The Institute of Medical Science, Tokyo University) for providing plasmids used for retroviral vector construction and PLAT-E cells; Professor Shin-ichi Nishikawa (Laboratory for Stem Cell Biology, the Center for Developmental Biology, RIKEN Center for Developmental Biology, Kobe, Japan) for providing the Avas12α1 hybridoma cells producing anti-flk1 mAb; Dr Hiromi Fujiwara for providing Meth-A cells; and Professor Nicholas P Restifo (National Cancer Institute) for providing CT26 cells. The present study was supported in part by grants from the Ministry of Health, Labor and Welfare of Japan; by a grant-in-aid for Scientific Research on Priority Areas (17016043) and Young Scientists (A) (18689007) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and by a grant from the Takeda Science Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kanagawa, N., Yanagawa, T., Nakagawa, T. et al. Tumor vessel-injuring ability improves antitumor effect of cytotoxic T lymphocytes in adoptive immunotherapy. Cancer Gene Ther 20, 57–64 (2013). https://doi.org/10.1038/cgt.2012.85
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2012.85
Keywords
This article is cited by
-
Interaction of Taspine Derivative TPD7 with Vascular Endothelial Growth Factor Receptor 2 by Cell Membrane Chromatography
Chromatographia (2019)
-
Immunological quality and performance of tumor vessel-targeting CAR-T cells prepared by mRNA-EP for clinical research
Molecular Therapy - Oncolytics (2016)