Abstract
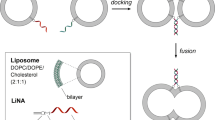
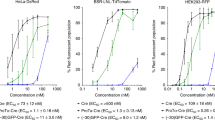
Multicomponent lipoplexes have recently emerged as especially promising transfection candidates, as they are from 10 to 100 times more efficient than binary complexes usually employed for gene delivery purposes. Previously, we investigated a number of chemical–physical properties of DNA–lipid complexes that were proposed to affect transfection efficiency (TE) of lipoplexes, such as nanoscale structure, size, surface potential, DNA-protection ability and DNA release from complexes upon interaction with cellular lipids. Although some minor differences between multicomponent and binary lipoplexes were found, they did not correlate clearly with efficiency. Instead, here we show that a marked difference between the cell internalization mechanism of binary and multicomponent lipoplexes does exist. Multicomponent lipoplexes significantly transfect cells at 4 °C, when endocytosis does not take place suggesting that they can enter cells via a temperature-independent mechanism. Confocal fluorescence microscopy experiments showed the existence of a correlation between endosomal escape and TE. Multicomponent lipoplexes exhibited a distinctive ability of endosomal escape and release DNA into the nucleus, whereas, poorly efficient binary lipoplexes exhibited minor, if any, endosomal rupture ability and remained confined in perinuclear late endosomes. Stopped-flow mixing measurements showed that the fusion rates of multicomponent cationic liposomes with anionic vesicles, used as model systems of cell membranes, were definitely shorter than those of binary liposomes. As either lipoplex uptake and endosomal escape involve fusion between lipoplex and cellular membranes, we suggest that a mechanism of lipoplex–cellular membrane interaction, driven by lipid mixing between cationic and anionic cellular lipids, does explain the TE boost of multicomponent lipoplexes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Niculescu-Duvaz I, Spooner R, Marais R, Springer CJ . Gene-directed enzyme prodrug therapy. Bioconjug Chem 1998; 9: 4–22.
Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000; 288: 669–672.
Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F . A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2003; 348: 255–256.
Marshall E . Gene therapy on trial. Science 2002; 288: 951–952.
Felgner PL, Ringold GM . Cationic liposome mediated transfection. Nature 1989; 331: 461–462.
Dass CR . Lipoplex-mediated delivery of nucleic acids: factors affecting in vivo transfection. J Mol Med 2004; 82: 579–591.
Harrington JJ, Van Bokkelen G, Mays RW, Gustashaw K, Willard HF . Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nat Genet 1997; 15: 345–355.
Willard HF . Artificial chromosomes coming to life. Science 2000; 290: 1308–1309.
Elouahabi A, Ruysschaert JM . Formation and intracellular trafficking of lipoplexes and polyplexes. Mol Ther 2005; 11: 336–347.
Medina-Kauwe LK, Xie J, Hamm-Alvarez S . Intracellular trafficking of nonviral vectors. Gene Therapy 2005; 12: 1734–1751.
Ewert K, Evans HM, Ahmad A, Slack NL, Lin AJ, Martin-Herranz A et al. Lipoplex structures and their distinct cellular pathways. Adv Genet 2005; 53: 119–155.
Zuhorn IS, Hoekstra D . On the mechanism of cationic amphiphile-mediated transfection. To fuse or not to fuse: is that the question? J Membr Biol 2002; 189: 167–179.
Hoekstra D, Rejman J, Wasungu L, Shi F, Zuhorn IS . Gene delivery by cationic lipids: in and out of an endosome. Biochem Soc Trans 2007; 35: 68–71.
Caracciolo G, Pozzi D, Caminiti R, Marchini C, Montani M, Amici A et al. Transfection efficiency boost by designer multicomponent lipoplexes. Biochim Biophys Acta 2007; 1768: 2280–2292.
Caracciolo G, Marchini C, Pozzi D, Caminiti R, Amenitsch H, Montani M et al. Structural stability against disintegration by anionic lipids rationalizes the efficiency of cationic liposome/DNA complexes. Langmuir 2007; 23: 4498–4508.
Caracciolo G, Caminiti R, Digman MA, Gratton E, Sanchez S . Efficient escape from endosomes determines the superior efficiency of multicomponent lipoplexes. J Phys Chem B 2009; 113: 4995–4997.
Caracciolo G, Pozzi D, Caminiti R, Marchini C, Montani M, Amici A et al. Enhanced transfection efficiency of multicomponent lipoplexes in the regime of optimal membrane charge density. J Phys Chem B 2008; 112: 11298–11304.
Radler JO, Koltover I, Salditt T, Safinya CR . Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science 1997; 275: 810–814.
Koltover I, Salditt T, Safinya CR . Phase diagram, stability, and overcharging of lamellar cationic lipid-DNA self-assembled complexes. Biophys J 1999; 77: 915–924.
Caracciolo G, Caminiti R . DNA–DNA electrostatic interactions within cationic lipid/DNA lamellar complexes. Chem Phys Lett 2004; 400: 314–319.
Kennedy MT, Pozharski EV, Rakhmanova VA, MacDonald RC . Factors governing the assembly of cationic phospholipid-DNA complexes. Biophys J 2000; 78: 1620–1633.
Pires P, Simões S, Nir S, Gaspar R, Düzgünes N, Pedroso de Lima MC . Interaction of cationic liposomes and their DNA complexes with monocytic leukemia cells. Biochim Biophys Acta 1999; 1418: 71–84.
Koltover I, Wagner K, Safinya CR . DNA condensation in two dimensions. Proc Natl Acad Sci 2000; 97: 14046–14051.
Wang L, Koynova R, Parikh H, MacDonald RC . Transfection activity of binary mixtures of cationic O-substituted phosphatidylcholine derivatives: the hydrophobic core strongly modulates their physical properties and DNA delivery efficacy. Biophys J 2006; 91: 3692–3706.
May S, Harries D, Ben-Shaul A . The phase behavior of cationic lipid-DNA complexes. Biophys J 2000; 78: 1681–1697.
May S, Ben-Shaul A . Modeling of cationic lipid-DNA complexes. Curr Med Chem 2004; 11: 1241–1253.
Marchini C, Montani M, Amici A, Pozzi D, Caminiti R, Caracciolo G . Surface area of lipid membranes regulates the DNA-binding capacity of cationic liposomes. Appl Phys Lett 2009; 94: 033903.
Rejman J, Oberle V, Zuhorn IS, Hoekstra D . Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J 2004; 377: 159–169.
Rejman J, Bragonzi A, Conese M . Role of clathrin- and caveolae mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther 2005; 12: 468–474.
Ortiz A, Killian JA, Verkleij AJ, Wilschut J . Membrane fusion and the lamellar-to-inverted-hexagonal phase transition in cardiolipin vesicle systems induced by divalent cations. Biophys J 1999; 77: 2003–2014.
de Lima MC, Simoes S, Pires P, Gaspar R, Slepushkin V, Duzgunes N . Gene delivery mediated by cationic liposomes: from biophysical aspects to enhancement of transfection. Mol Membr Biol 1999; 16: 103–109.
Top D, de Antueno R, Salsman J, Corcoran J, Mader J, Hoskin D et al. Liposome reconstitution of a minimal protein-mediated membrane fusion machine. EMBO J 2005; 24: 2980–2988.
Goryacheva YA, Vekshina OM, Yashin VA, Kim YA . Fusion and endocytosis of anionic liposomes with Ehrlich ascitic carcinoma cells. Bull Exp Biol Med 2005; 140: 733–735.
Resina S, Prevot P, Thierry AR . Physico-chemical characteristics of lipoplexes influence cell uptake mechanisms and transfection efficacy. PloS ONE 2009; 4: e6058.
Pozzi D, Caracciolo G, Caminiti R, Candeloro De Sanctis S, Amenitsch H, Marchini C et al. Toward the rational design of lipid gene vectors: shape coupling between lipoplex and anionic cellular lipids controls the phase evolution of lipoplexes and the efficiency of DNA release. ACS Appl Mat Int 2009; 10: 2237–2249.
Elouahabi A, Ruysschaert J . Formation and intracellular trafficking of lipoplexes and polyplexes. Mol Ther 2005; 11: 336–347.
Zuhorn IS, Visser WH, Bakowsky U, Engberts JB, Hoekstra D . Interference of serum with lipoplex–cell interaction: modulation of intracellular processing. Biochim Biophys Acta 2002; 1560: 25–36.
Walter A, Siegel DP . Divalent cation-induced lipid mixing between phosphatidylserine liposomes studied by stopped-flow fluorescence measurements: effects of temperature, comparison of barium and calcium, and perturbation by DPX. Biochemistry 1993; 32: 3271–3281.
Evans KO, Lentz BR . Kinetics of lipid rearrangements during poly(ethylene glycol)-mediated fusion of highly curved unilamellar vesicles. Biochemistry 2002; 41: 1241–1249.
Lu JJ, Langer R, Chen J . A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol Pharmaceutics 2009; 6: 763–771.
Stamatatos L, Leventis R, Zuckerman MJ, Silvius JR . Interactions of cationic lipid vesicles with negatively charged phospholipid vesicles and biological membranes. Biochemistry 1988; 27: 3917–3925.
Duzgunes N, Goldstein JA, Friend DS, Felgner PL . Fusion of liposomes containing a novel cationic lipid, N-[2,3-(dioleyloxy)propyl]-N,N,N-trimethylammonium: Induction by multivalent anions and asymmetric fusion with acidic phospholipid vesicles. Biochemistry 1989; 28: 9179–9184.
Mönkkönen J, Urtti A . Lipid fusion in oligonucleotide and gene delivery with cationic lipids. Adv Drug Deliv Rev 1998; 34: 37–49.
Harashima H, Shinohara Y, Kiwada H . Intracellular control of gene trafficking using liposomes as drug carriers. Eur J Pharm Sci 2001; 13: 85–89.
Zuhorn IS, Kalicharan R, Hoekstra D . Lipoplex-mediated transfection of mammalian cells occurs through the cholesterol-dependent clathrin-mediated pathway of endocytosis. J Biol Chem 2002; 277: 18021–18028.
Chernomordik LV, Zimmerberg J . Bending membranes to the task: structural intermediates in bilayer fusion. Curr Opin Struct Biol 1995; 5: 541–547.
Soriano P, Dijkstra J, Legrand A, Spanjer H, Londos-Gagliardi D, Roerdink F et al. Targeted and nontargeted liposomes for in vivo transfer to rat liver cells of a plasmid containing the preproinsulin I gene. Proc Natl Acad Sci USA 1983; 80: 7128–7131.
Ciftci K, Levy RJ . Enhanced plasmid DNA transfection with lysosomotropic agents in cultured fibroblasts. Int J Pharm 2001; 218: 81–92.
Xu Y, Szoka FC . Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 1996; 35: 5616–5623.
Jahn R, Südhof TC . Membrane fusion and exocytosis. Annu Rev Biochem 1999; 68: 863–911.
Caracciolo G, Pozzi D, Caminiti R, Amenitsch H . Two-dimensional lipid mixing entropy regulates the formation of multicomponent lipoplexes. J Phys Chem B 2006; 110: 20829–20835.
Caracciolo G, Pozzi D, Amenitsch H, Caminiti R . Multicomponent cationic lipid-DNA complex formation: role of lipid mixing. Langmuir 2005; 21: 11582–11587.
Caracciolo G, Pozzi D, Caminiti R, Amenitsch H . Lipid mixing upon deoxyribonucleic acid-induced liposomes investigated by synchrotron small-angle X-ray scattering. Appl Phys Lett 2005; 87: 133901.
Acknowledgements
This work was supported in part by U54 GM064346 Cell Migration Consortium (MD and EG), NIH-P41 p41-RRO3155 (for example, SS) and P50-GM076516 grant (EG). We thank Dr Giulia Ossato for helping with the cell cultures. Dr Massimiliano Papi is acknowledged for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Cancer Gene Therapy website
Rights and permissions
About this article
Cite this article
Marchini, C., Pozzi, D., Montani, M. et al. Role of temperature-independent lipoplex–cell membrane interactions in the efficiency boost of multicomponent lipoplexes. Cancer Gene Ther 18, 543–552 (2011). https://doi.org/10.1038/cgt.2011.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2011.12
Keywords
This article is cited by
-
Effect of DOPE and cholesterol on the protein adsorption onto lipid nanoparticles
Journal of Nanoparticle Research (2013)
-
Cationic liposome/DNA complexes: from structure to interactions with cellular membranes
European Biophysics Journal (2012)